Interleukin-4 therapeutic vaccine for treating immune related diseases of humans or animals
A therapeutic vaccine and interleukin technology, applied in the field of interleukin-4 therapeutic vaccine, can solve problems such as increasing costs, easily causing allergic reactions, and short half-life of antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Construction and vaccine preparation of mouse IL-4 gene recombinant vaccine
[0016] According to the whole gene coding sequence of mouse IL-4, a pair of suitable primers were designed to amplify IL-4 cDNA sequence by RT-PCR method. Then, after the DNA sequence was determined to prove that the amplified cDNA sequence was correct, it was cloned into the appropriate part of the pET-32 fusion expression vector, and the recombinant plasmid and the pET-32 fusion expression vector blank control plasmid were further transferred into Escherichia coli strains for proper expression were used for large-scale fermentation to extract and purify the IL-4 fusion protein and the thioredoxin expressed by the vector control. After mixing the purified IL-4 fusion protein or the thioredoxin expressed by the carrier control with oil adjuvant or aluminum gel adjuvant in an appropriate proportion [according to the content of the purified IL-4 fusion protein, for oil adjuvant vaccin...
Embodiment 2
[0019] Example 2: Safety test and allergic reaction test of mouse IL-4 gene recombinant vaccine
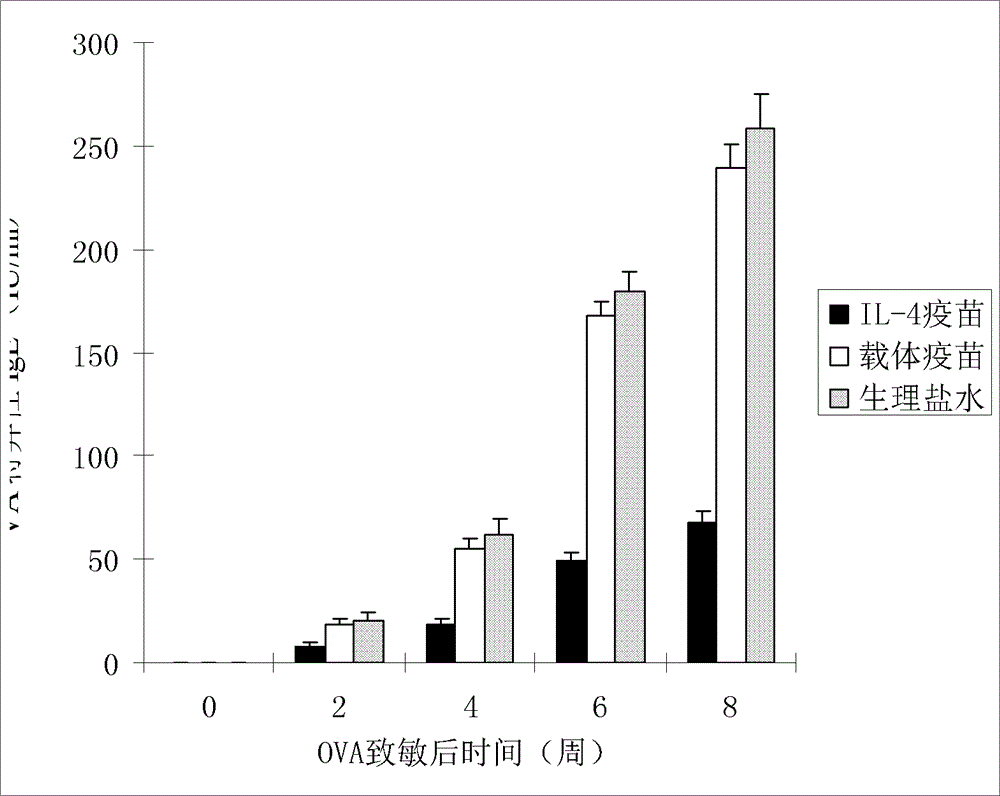
[0020] According to the method of Example 1, the purified IL-4 fusion protein or the thioredoxin expressed by the carrier control was properly diluted with physiological saline and then mixed with oil adjuvant or aluminum gel adjuvant at a volume ratio of 1:1 to prepare IL -4 fusion protein vaccine or vehicle control thioredoxin vaccine. The prepared IL-4 fusion protein vaccine was divided into three different dose groups of low (0.1ml, about 1 dose), medium (0.25ml, about 2.5 doses), high (0.5ml, about 5 doses) to immunize BALB respectively / c mice, pET-32 vector vaccine and normal saline control group were set up separately, 5 mice in each group, injected subcutaneously at multiple points. The second and third immunization injections were performed at intervals of 3 weeks. After the vaccine was injected, the mice were observed for adverse reactions, allergies, and death. The ...
Embodiment 3
[0021] Embodiment 3: The test of enhancing anti-tumor activity of mouse IL-4 gene recombinant vaccine
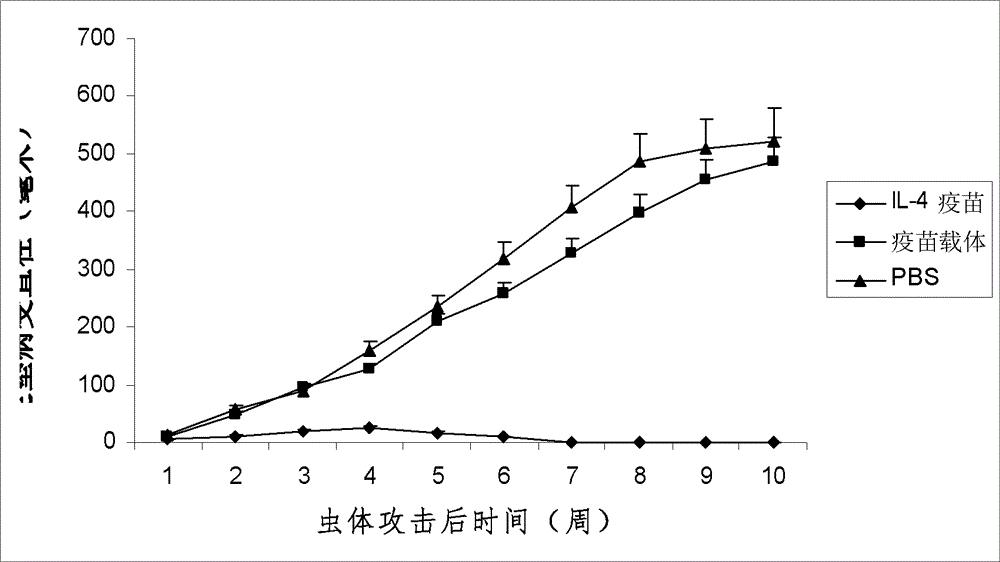
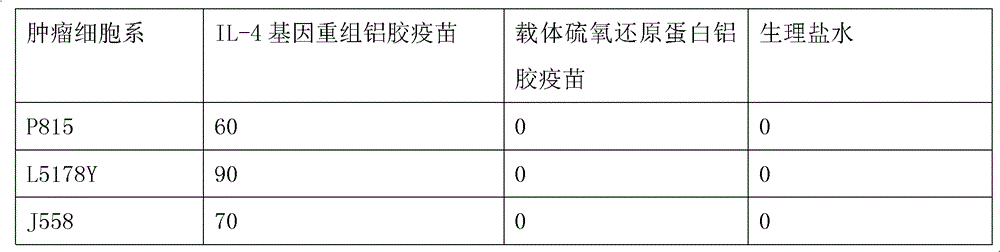
[0022] According to the method of Example 1, the purified IL-4 fusion protein or the thioredoxin expressed by the carrier control was properly diluted with physiological saline and then mixed with aluminum gel adjuvant at a ratio of 1:1 to prepare the experimental or control vaccine. It is required that the amount of IL-4 fusion protein or carrier control thioredoxin in each vaccine is about 15-20 μg; the volume of each vaccine is about 0.1 ml. The experimental mice were inoculated intraperitoneally (i.p.) with the IL-4 gene recombinant aluminum gel vaccine prepared in Example 1 3 weeks and 1 week before the tumor cell attack, respectively, and a pET-32 carrier aluminum gel vaccine and normal saline immunized control group were also set up . Tumor growth was checked every 3 days after tumor inoculation, and the experiment ended at 60 days. The results showed that the IL-4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com