Neurotensin-derived branched peptides and uses thereof
A use and molecular technology, applied in the field of branched peptides, can solve problems such as insufficient activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
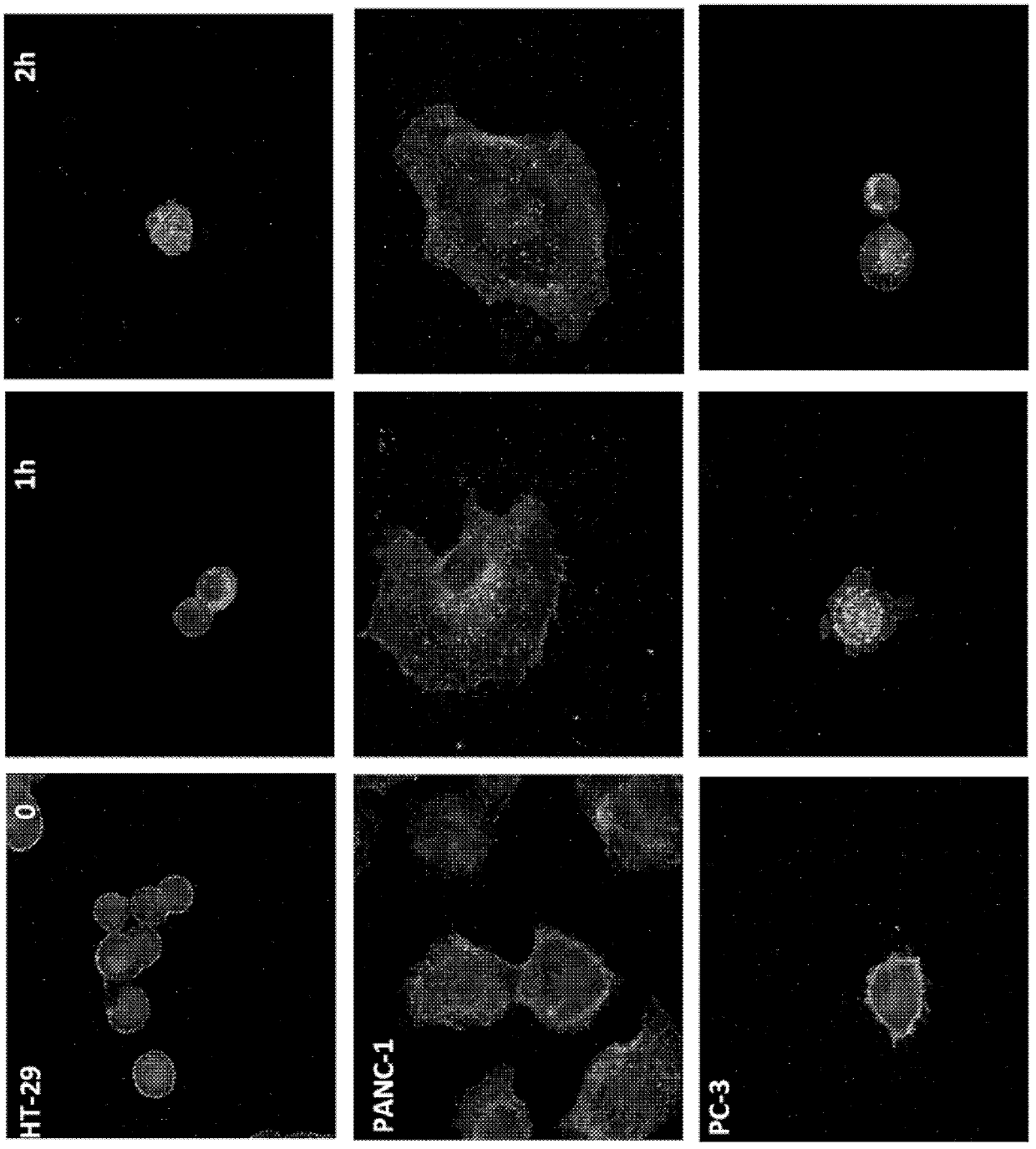
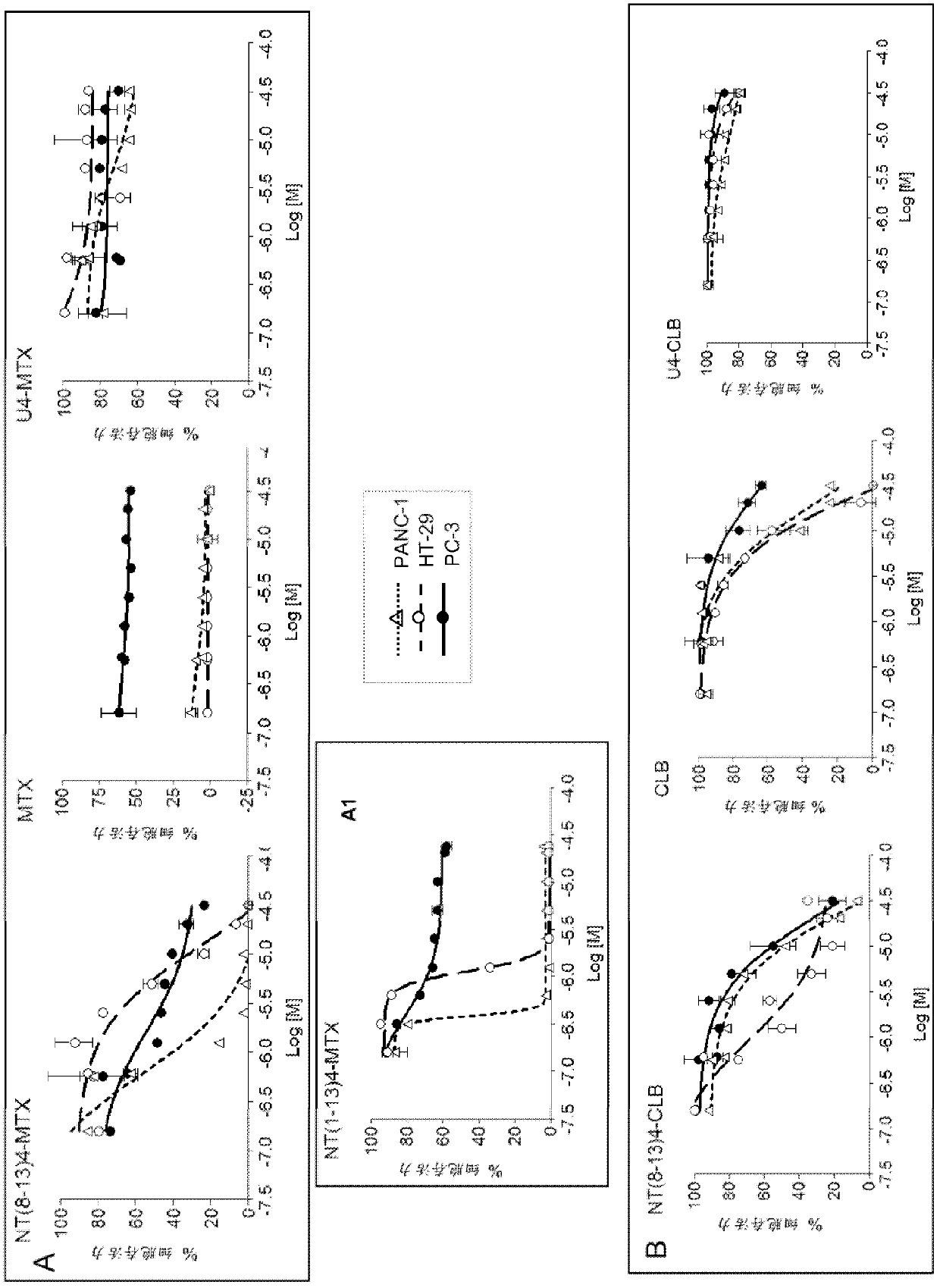
Image
Examples
Embodiment Construction
[0040] peptide synthesis
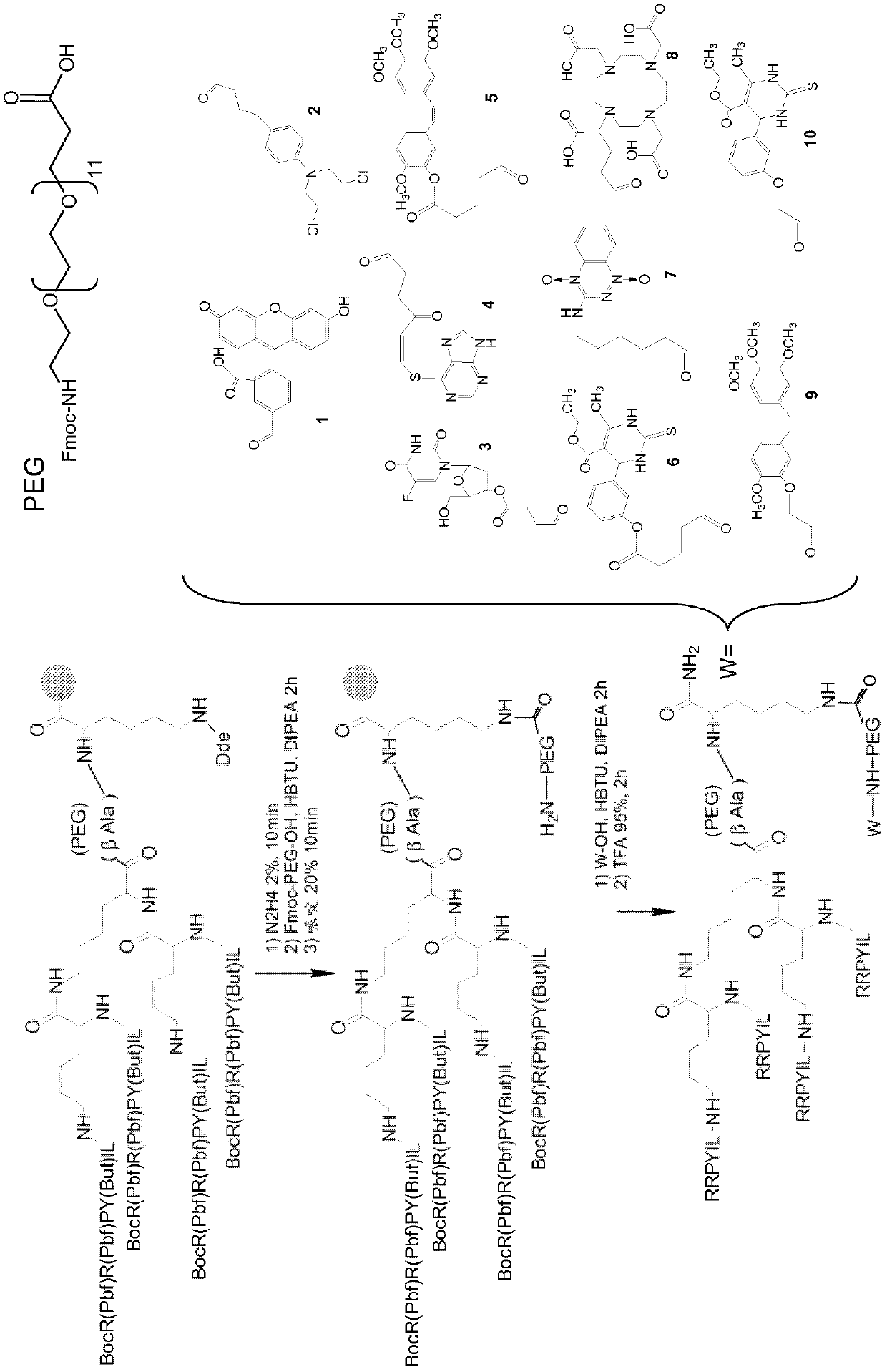
[0041] Tetrabranched NT(8-13)-PEG-K(PEG_fluorescein)[NT4(8-13)-Fluo]1, NT(8-13)-PEG-chlorambucil[NT4 (8-13)-CLB] 2, four-branched NT(8-13)-PEG-5-fluorodeoxyuridine [NT4(8-13)-5-FdU]3, four-branched NT(8 -13)-PEG-6-mercaptopurine [NT4(8-13)-6-MP]4, four-branched NT(8-13)-PEG-combretastatin [NT4(8-13)-CBTST] 5. Four-branched NT(8-13)-PEG-monostin [NT4(8-13)-Mon] 6. Four-branched NT(8-13)-PEG-tirapazamine [NT4( 8-13)-TPZ]7, four-branched NT(8-13)-PEG-DOTA [NT4(8-13)-DOTA]8 and four-branched NT(8-13)-PEG-DOTA Statin ether [NT4(8-13)-O-CBTST]9, and tetrabranched NT(8-13)-PEG-monostar ether [NT4(8-13)-O-Mon]10( figure 1 ), Fmoc-Lys(Dde)-OH applied on Novasyn TGR resin as the first amino acid and β-Ala as the second amino acid, wherein the second amino acid of [NT4(8-13)-Fluo]1 is Fmoc-PEG -OH, instead of β-Ala. The tetramer was then constructed as above, but with Boc-Arg(Pbf)-OH as the last amino acid of the neurotensin sequence, so that the last two ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com