Synthetic technology of ascorbic acid derivative
A synthesis process and technology of vitamins, applied in the field of pharmaceutical processing, can solve the problems of affecting the effective utilization rate and instability of vitamin C
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0012] Processing step of the present invention is:
[0013] 1. Synthesis of 5,6-O-isopropylidene-vitamin C
[0014] Add 100-150ml of acetone to a 250ml three-neck flask equipped with a reflux condenser, turn on the constant temperature water bath and electric stirrer, and then quickly add quantitative vitamin C so that the amount of acetone and vitamin C is 10:1-20: 1. Add 5-10ml of catalyst hydroiodic acid, heat and stir at 25-30°C for 2-3 hours, filter under reduced pressure after the reaction, wash the product repeatedly with acetone and n-hexane, and dry it in vacuum;
[0015] 2. Synthesis of Magnesium Ascorbyl Phosphate
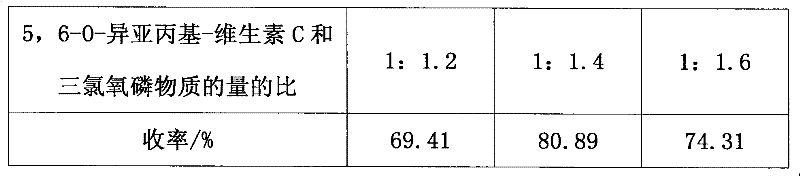
[0016] Add 8:1~10:1 mixed solution of distilled water and pyridine into a 250ml four-necked flask, then place it in an ice-water bath and stir, protect with nitrogen, then add 5,6-O-isopropylidene-vitamin C, and use Add phosphorus oxychloride dropwise to the dropping funnel, so that the ratio of the amount of 5,6-O-isopropylidene-vitamin C and phospho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com