Neutralizing prolactin receptor antibodies and their therapeutic use
A prolactin receptor and antibody technology, applied in the direction of antibody medical components, antibodies, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve the problem of interfering with PRLR-mediated signal transduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0378] Example 1 Isolation of target-specific antibodies from a human antibody phage display library
[0379] To isolate a panel of antibodies capable of neutralizing the activity of human PRLR, three human antibody phage display libraries expressing Fab and scFv fragments were studied in parallel. The target used for library panning was the soluble extracellular domain (ECD) of the prolactin receptor (human prolactin receptor amino acids 25-234) prepared as described above in WO08 / 022295 (Novartis). Alternative targets are the ECD of PRLR, whose C-terminus is linked to 6 histidines or via an amino acid sequence "isoleucine-glutamic acid-glycine-arginine-methionine-aspartic acid" The linker was attached to the human IgG1-Fc domain.
[0380] Selection of target-specific antibodies from phage display was performed according to the method described by Marks et al. (Methods MoI Biol. 248:161-76, 2004). Briefly, phage display libraries were incubated with 50 pmol biotinylated ECD...
Embodiment 2
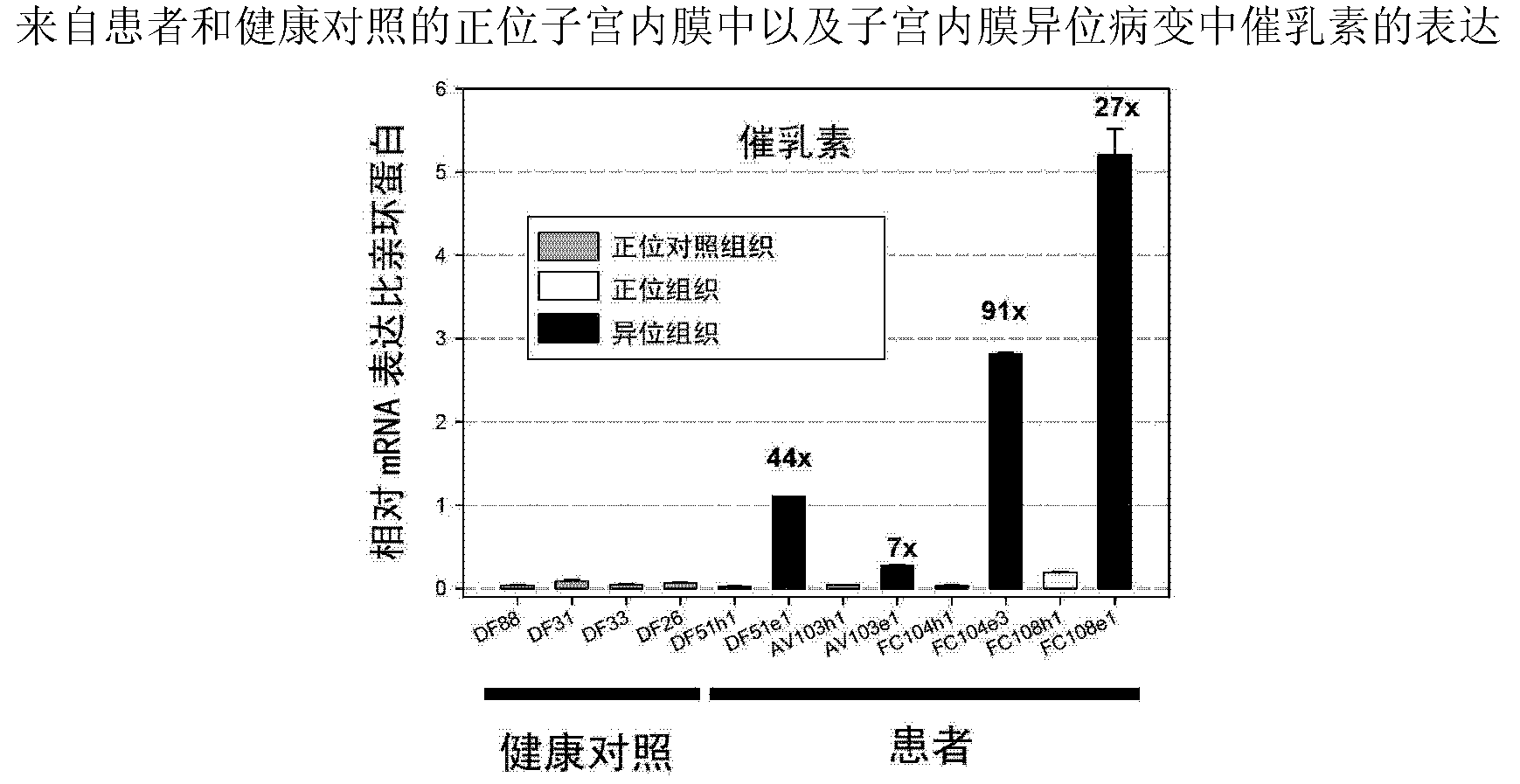
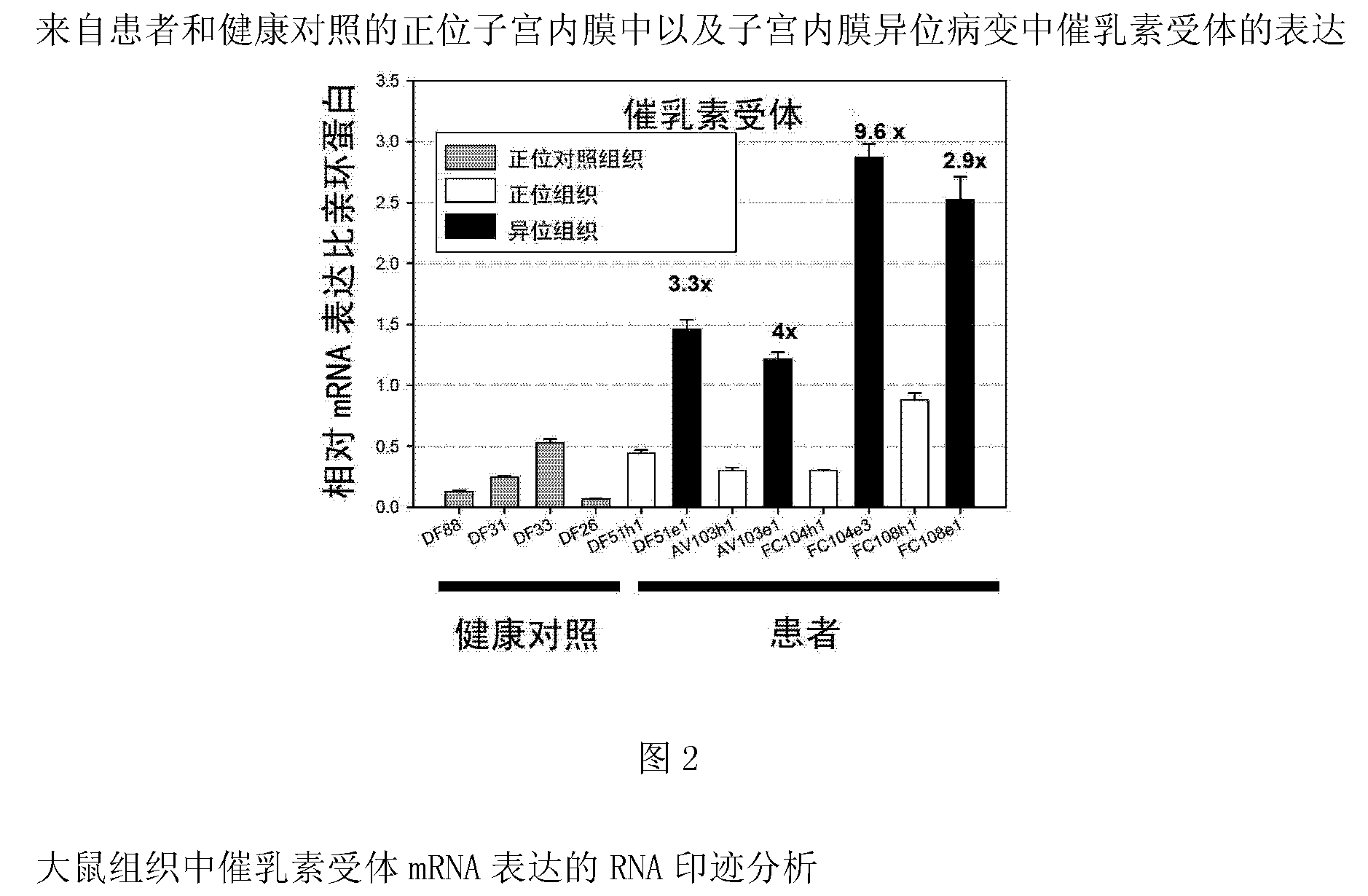
[0383] Example 2 Quantitative Analysis of Prolactin and Prolactin Receptor Gene Expression in Orthotopic and Ectopic Endometrium and Endometriosis Lesions from Patients and Healthy Controls by Real-Time TaqMan PCR Analysis
[0384] Real-time Taqman PCR analysis was performed using the ABI Prism 7700 Sequence Detector System according to the manufacturer's instructions (PE Applied Biosystems) and as described (Endocrinolgy 2008, 149(8):3952-3959) and known to those skilled in the art . Relative expression levels of PRL and PRLR were normalized to cyclophyllin expression. We utilized quantitative real-time Taqman PCR analysis to analyze the expression of PRL and PRLR in endometrium from healthy women as well as endometrium and endometriotic lesions from patients. The expression of prolactin and its receptor is significantly upregulated in endometriotic lesions compared with healthy endometrium or endometrium derived from patients.
[0385] The result is as figure 1 and 2 sh...
Embodiment 3
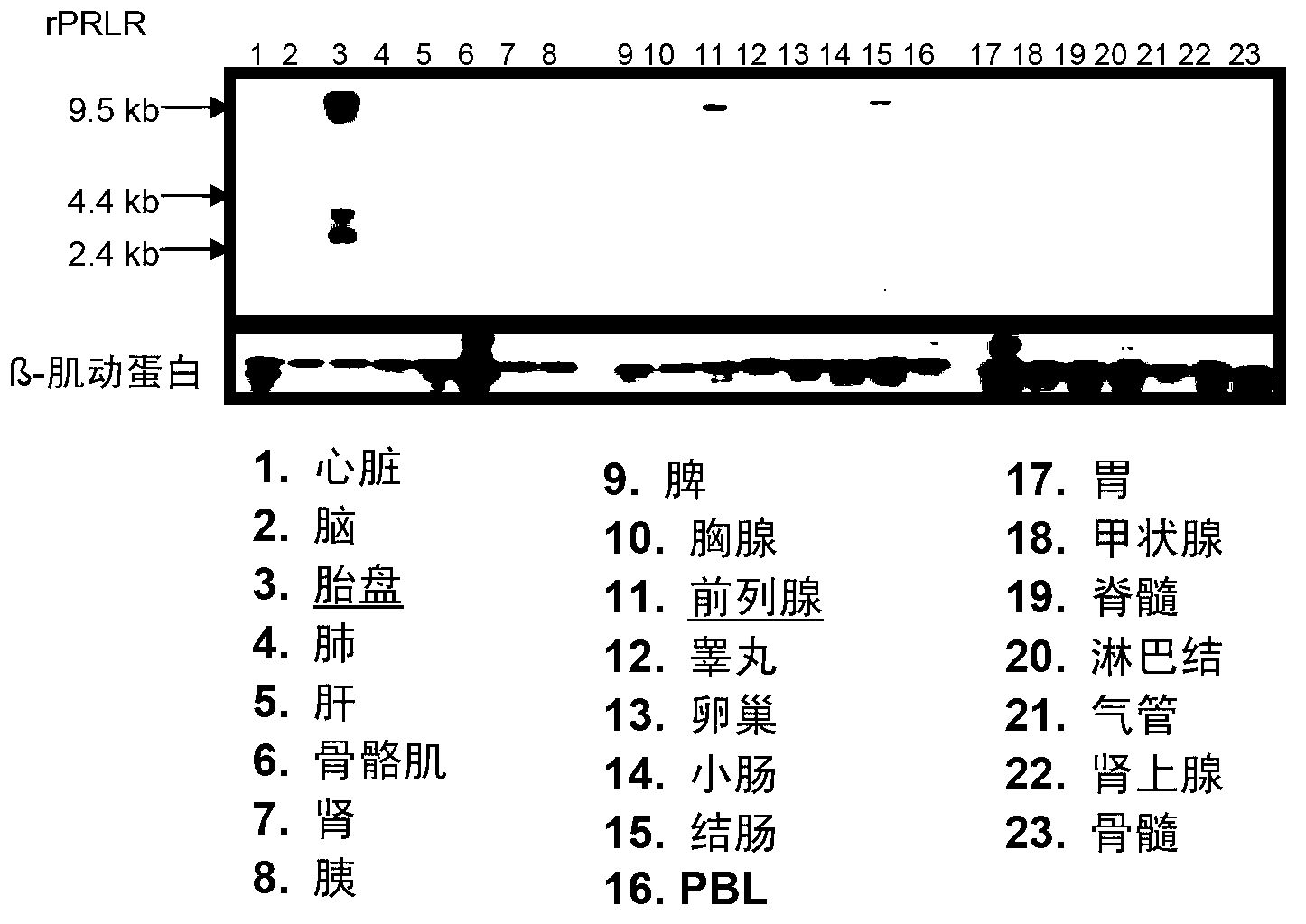
[0387] Example 3 Analysis of Prolactin Receptor Expression in Human Tissues by Northern Blot
[0388] RNA was isolated from different rat tissues and transferred to nylon membranes after gel electrophoresis. The membrane was sequentially hybridized with radiolabeled cDNA of rat prolactin receptor or β-actin (as a loading control), washed and exposed to film. Bands correspond to rat prolactin receptor and β-actin mRNAs. image 3 The results shown show strong expression of prolactin receptors in the placenta, prostate, ovary and adrenal gland.
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com