Liposome containing shRNA molecule for thymidylate synthase, and use for same
A technology of thymidylate synthase and plastids, which is applied in the field of chemotherapy drugs, can solve the problems of side effects and inability to obtain sufficient effects, and achieve the effect of inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1: Preparation of RNAi molecules
[0092] The following siRNA and shRNA were synthesized according to well-known conventional methods.
[0093] (I) siRNA targeting TS
[0094] The siRNA targeting TS was synthesized based on the anti-TS siRNA (WO2010 / 113844) whose antitumor effect was confirmed, and consisted of the following sense strand and antisense strand.
[0095] Sense strand:
[0096] 5'-GUAACACCAUCGAUCAUGA-3' (SEQ ID NO: 1)
[0097] Antisense strand:
[0098] 5'-UCAUGAUCGAUGGUGUUAC-3' (SEQ ID NO: 2)
[0099] Hereinafter, siRNA targeting TS is referred to as "siTS".
[0100] (II) siRNA targeting luciferase
[0101] As a control siRNA, siRNA targeting luciferase was synthesized. This siRNA consists of a sense strand and an antisense strand as described below.
[0102] Sense strand:
[0103] 5'-CUUACGCUGAGUACUUCGATT-3' (SEQ ID NO: 9)
[0104] Antisense strand:
[0105] 5'-UCGAAGUACUCAGCGUAAGTT-3' (SEQ ID NO: 10)
[0106] Hereinafter, siRNA targ...
Embodiment 2
[0112] Example 2: Inhibition of TS expression by siRNA and shRNA
[0113]
[0114] Lipofectamine, a type of cationic liposome, is used as a transfection reagent TM RNAi MAX (hereinafter referred to as "Lf RNAi MAX").
[0115] The shRNA or siRNA prepared in Example 1 and Lf RNAi MAX were diluted and mixed with OptiMEM respectively, so that the ratio of shRNA or siRNA and Lf RNAi MAX reached 100 (pmol): 5( mu L). At this time, the amount of shRNA or siRNA solution is equal to that of Lf RNAi MNX solution. A complex (lipoplex) is formed by allowing the mixed solution to stand at room temperature for 10 to 20 minutes.
[0116] Each lipoplex was directly added to a 10 cm Petri dish previously filled with OptiMEM, and adjusted so that the total volume became 5 ml. Next, 10 ml of DLD-1 or DLD-1 / FU cell suspension was inoculated in the culture dish so as to reach 500,000 cells / dish, and the final total volume was 15 ml for transfection. At this time, adjust so that the fina...
Embodiment 3
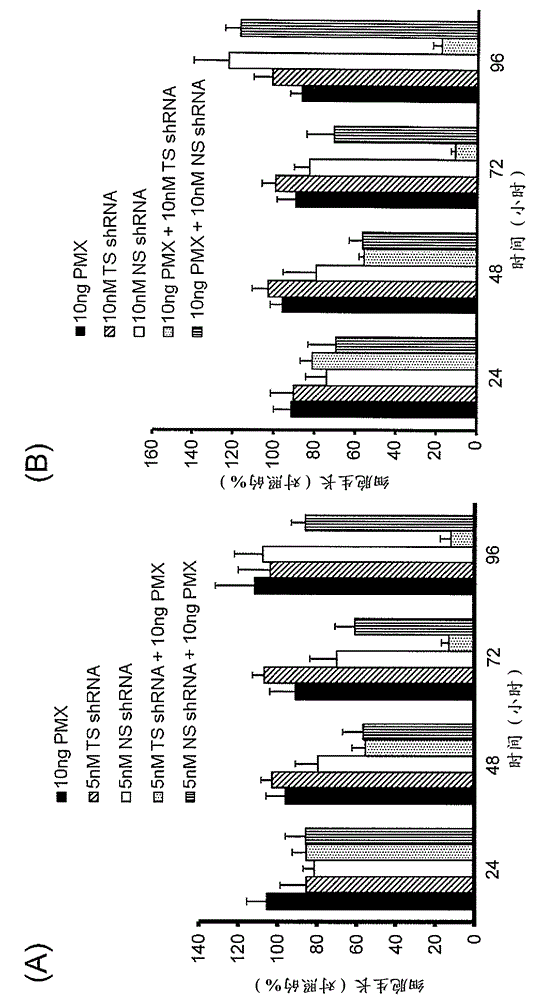
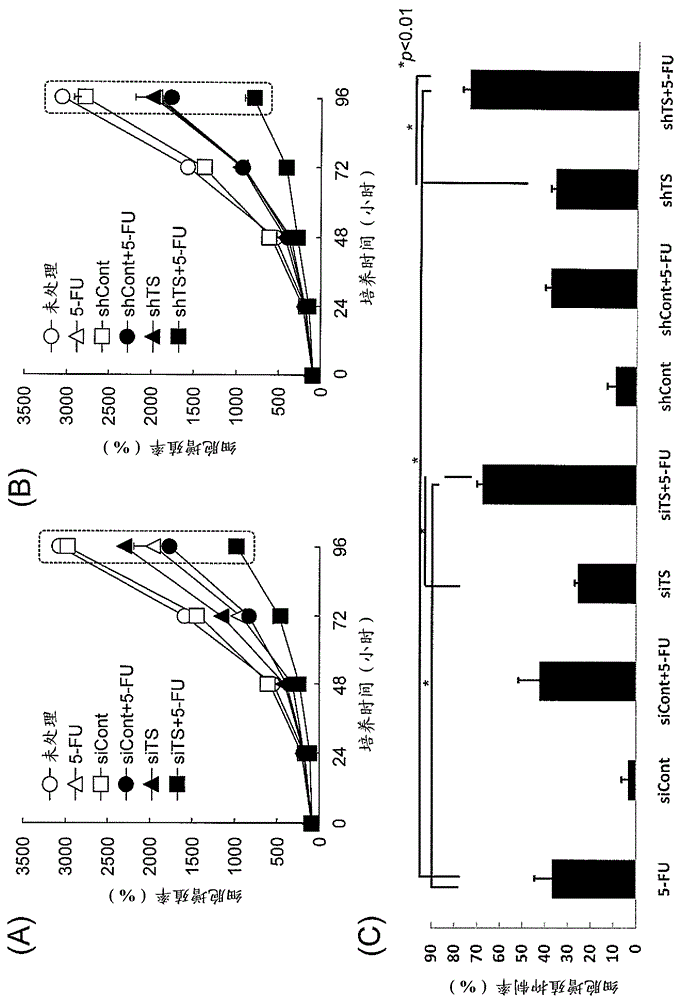
[0128] Example 3 : Cancer cell (human colon adenocarcinoma) growth inhibitory effect of siRNA and shRNA
[0129] In this experiment, experiments were performed on a 96-well plate scale. The lipoplex prepared in the same way as in Example 2 above was directly added to the wells previously equipped with OptiMEM, and the total volume reached 50 mu l. Next, the cell suspension of human colon adenocarcinoma cells DLD-1 or DLD-1 / FU (2,000 cells / 100 mu l) Add in wells containing lipoplex (final total volume of 150 mu l), carry out transfection. Here, the final concentration of shRNA or siRNA in the wells was 5 nM.
[0130] 24 hours after the start of transfection, the medium was removed and 200 mu l New media with or without the existing chemotherapeutic drug 5-FU (fluorouracil). Here, relative to DLD-1, with 0.1 mu Add 5-FU at a concentration of g / mL, relative to DLD-1 / FU, at 10 mu 5-FU was added at a concentration of g / mL. Add new medium, remove medium after 0, 24, 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com