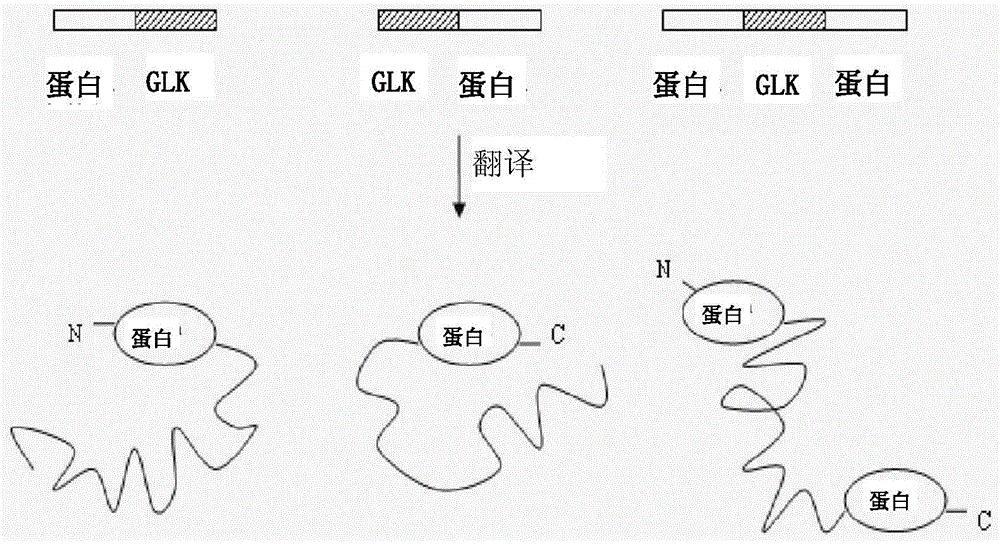
Use of gelatin-like units
A gelatin-like, purpose-built technology, applied in the protein field, can solve the problems of inability to express the obtained sequence, difficult to predict the spatial structure of the fully artificially designed sequence, and low actual expression.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0112] Preparation of recombinant gelatin-like fusion protein
[0113] The fusion protein of the present invention can be produced by direct synthesis of peptides using solid-phase technology, or the fragments of the protein of the present invention can be chemically synthesized separately and then chemically linked to produce full-length molecules. In a preferred embodiment, the fusion protein of the present invention is produced by recombinant methods.
[0114] The recombinant method to prepare the gelatin fusion protein involves expressing the nucleotides encoding the recombinant target gelatin fusion protein in prokaryotic hosts, eukaryotic hosts, plants or animals, and obtaining the recombinant gelatin-like fusion protein. Any system capable of expressing recombinant proteins, including prokaryotic, eukaryotic, and transgenic animal and plant systems, can be used in the present invention. For example, all the methods for expressing fusion proteins mentioned in US Pat. No...
Embodiment 1
[0159] Example 1: rGLK116 4 protein expression, purification
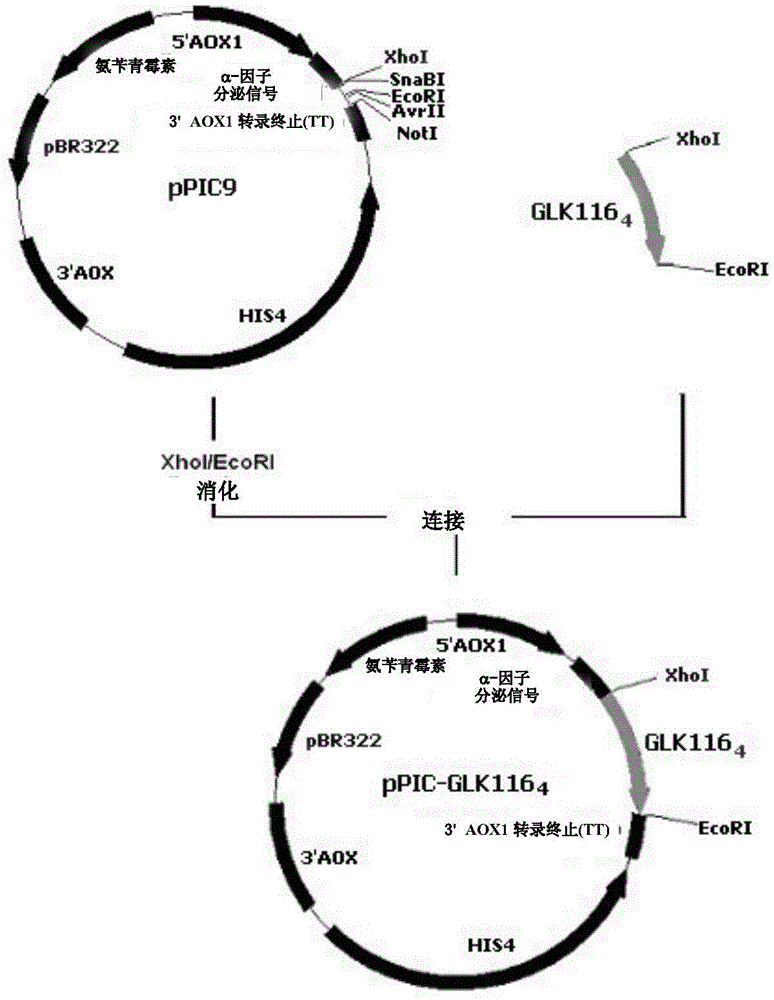
[0160] 1. GLK116 4 gene cloning
[0161] GLK116 in the present invention 4 The gene is composed of 4 identical monomers (see SEQ ID NO:1 for the sequence) in series, and the monomer is named GLK116 1 , encoding 116 amino acids (see SEQ ID NO: 2 for the sequence), synthesized by Shanghai Yingjun Biotechnology Co., Ltd. (Invitrogen). During synthesis, the α-factor signal peptide sequence of yeast GS115 (position 1-24 in SEQ ID NO:1, with Xho I site) was added to the 5' end, followed by the recognition site of endonuclease DraIII, 3 The 'end has Van91I and EcoRI recognition sites, and is connected to the cloning vector pMD18-T (TaKaRa Company) to construct the plasmid pGLK116 1 -T.
[0162] In order to obtain GLK116 2 Dimer, first the plasmid pGLK116 1 -T was digested with Van91I / Dra III. Electrophoresis was performed on 1% agarose gel, and the target fragment of about 330bp size (ie GLK116 1 ), purified wit...
Embodiment 2
[0178] Example 2: rGLK116 4 Expression, purification and identification of / G-CSF fusion protein
[0179] 1. Synthesis of hG-CSF gene
[0180] The hG-CSF gene (see SEQ ID NO: 4 for the sequence) was synthesized by Shanghai Zeheng Biotechnology Co., Ltd., and cloned into the pMD18-T vector to construct the plasmid pG-CSF-T. The 5' end of G-CSF is the DraIII recognition site, and the 3' end is the EcoRI recognition site.
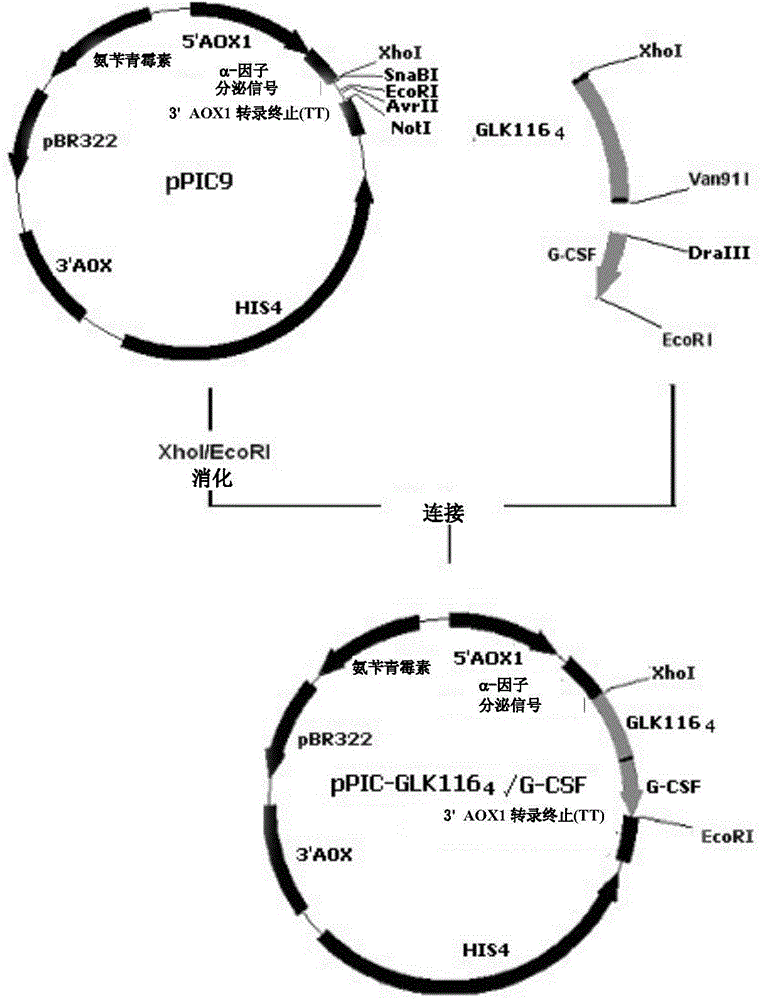
[0181] 2. Expression plasmid pPIC-GLK116 4 / G-CSF Construction
[0182] Basically the same as Example 1, the construction process of the expression plasmid can be found in image 3 , GLK116 4 / G-CSF DNA coding sequence and mature GLK116 4 See SEQ ID NO:5 and SEQ ID NO:6 for the amino acid sequences of the / G-CSF fusion protein, respectively.
[0183] 3. rGLK116 4 Construction of engineered yeast expressing / G-CSF fusion protein
[0184]pPIC-GLK116 4 / G-CSF transformed methanolic yeast Pichia pastor GS115 (His - ), the plasmid linearization treatment...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap