Use of 1h-quinazoline- 2, 4 -diones for use in the prevention or treatment photosensitive epilepsy
A quinazoline, photosensitivity technology for pharmaceutical applications in the treatment of photosensitive epilepsy (PSE), capable of addressing birth defects and more
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
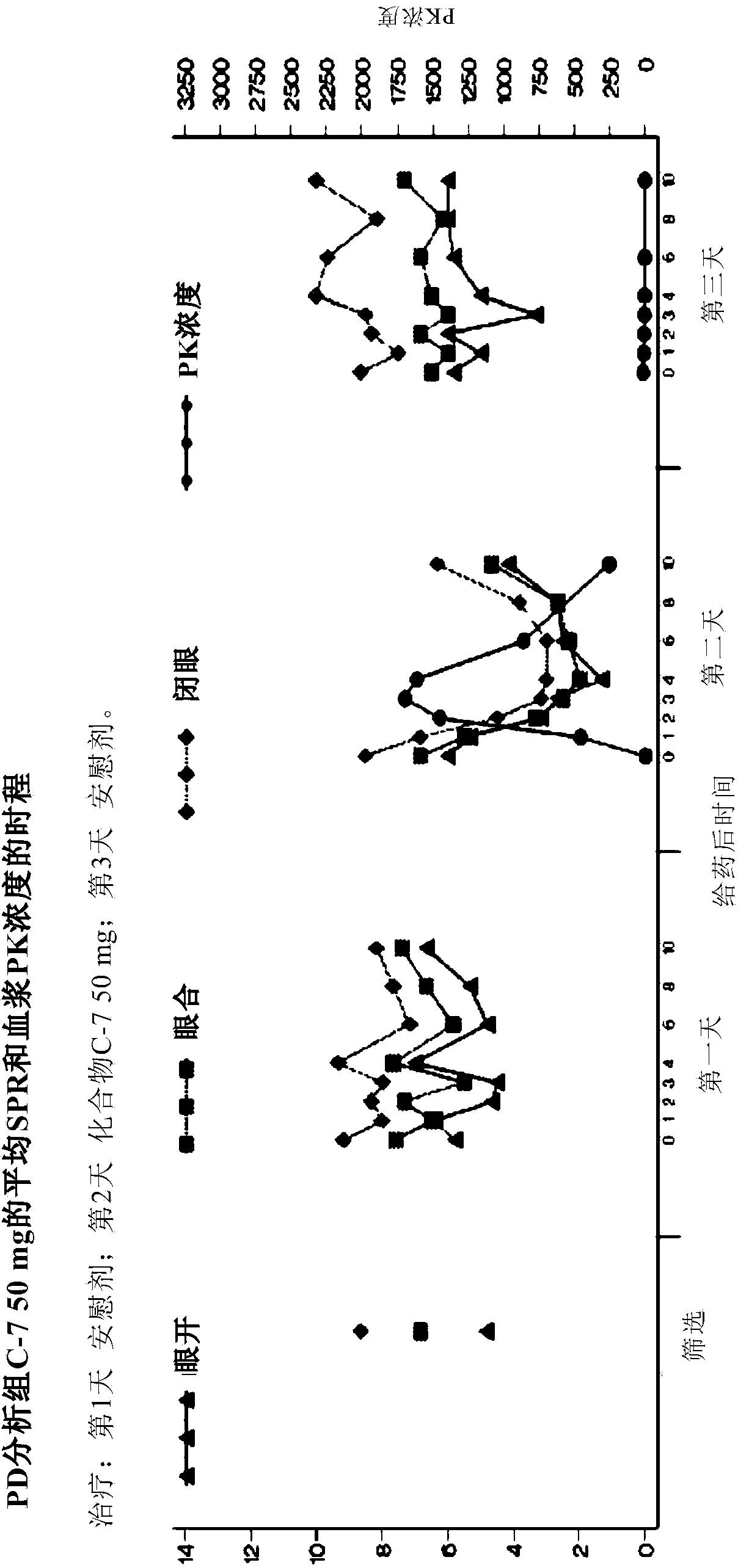
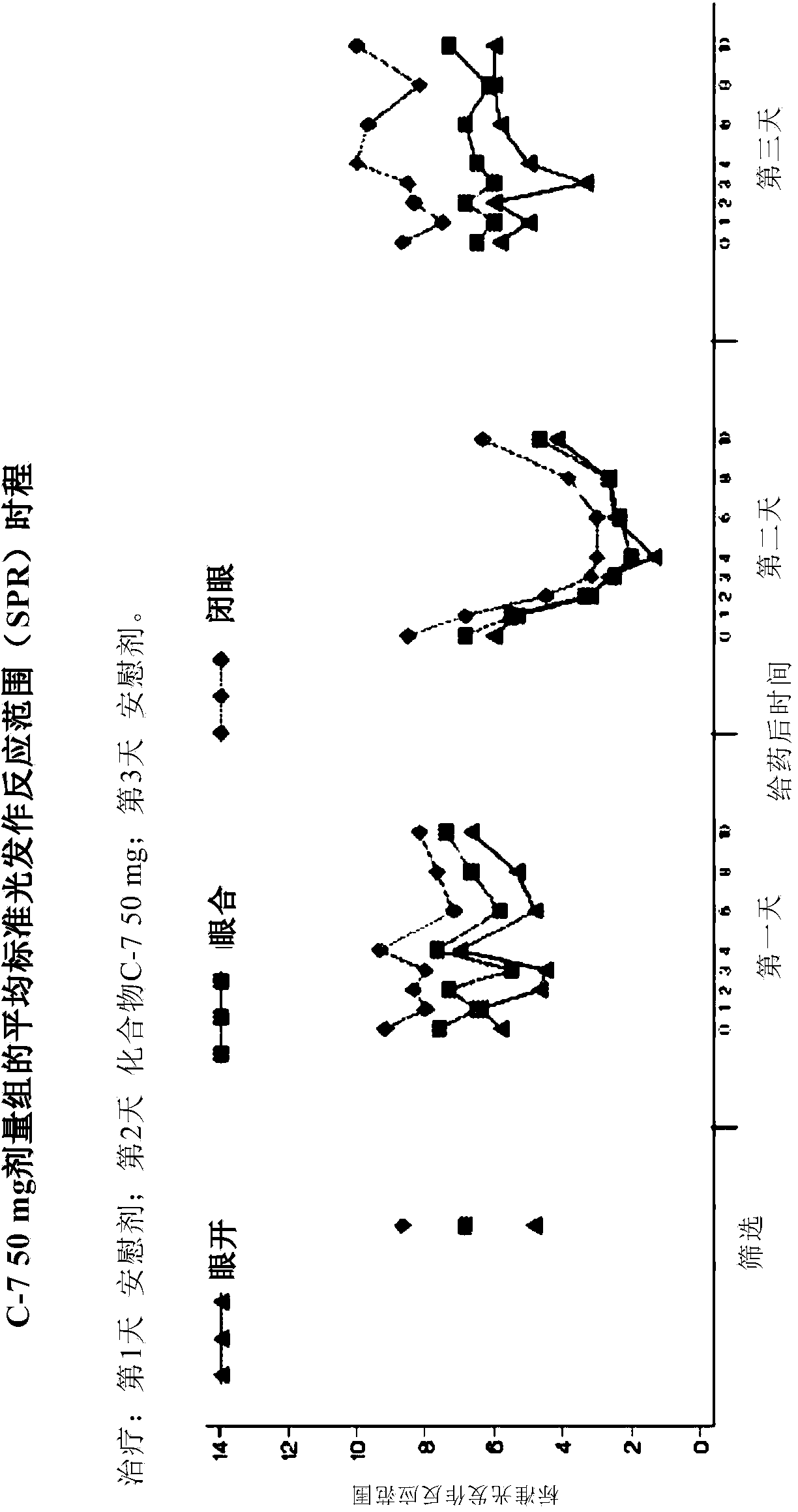
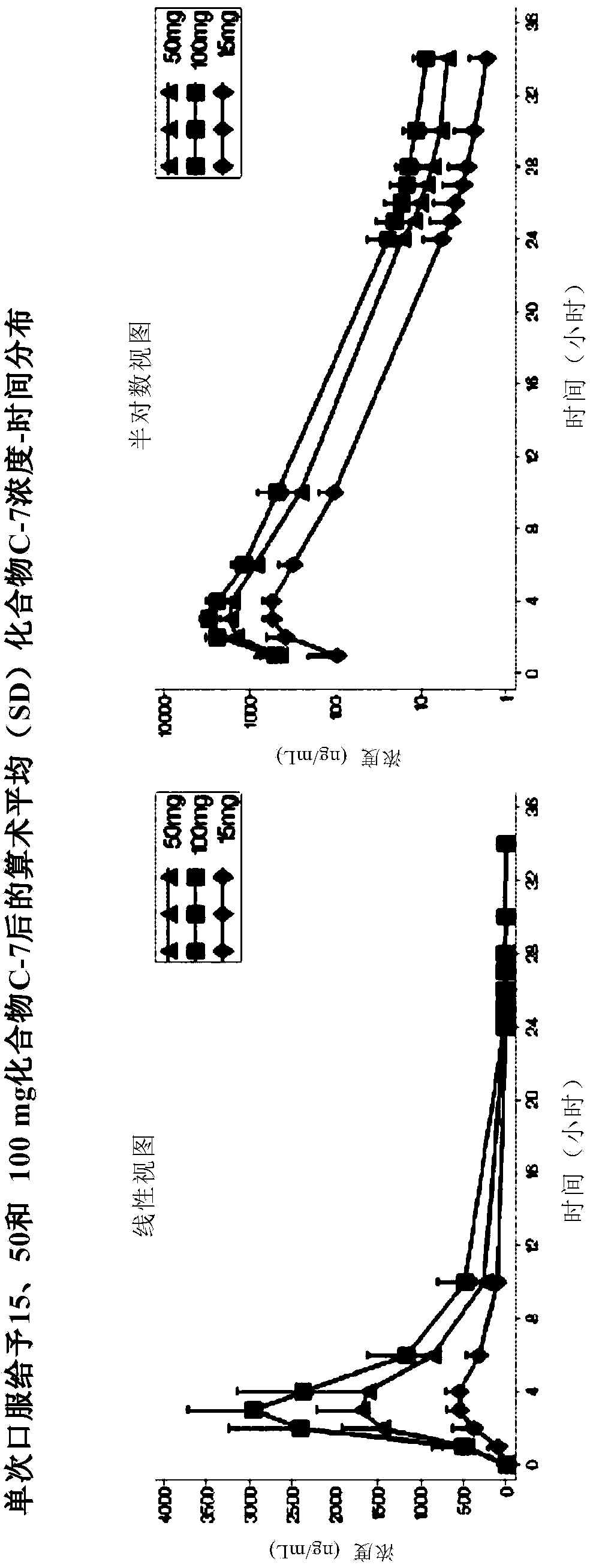
[0165] Compound C-7, used in the studies described below, is an orally active compound of general formula (I).
[0166]The study used the IPS / PPR paradigm. This is a multicenter, non-randomized, single-blind, within-subject, placebo-controlled proof-of-concept study conducted to evaluate a single oral dose of Compound C-7 in inhibiting photoictal responses in PSE patients ( PPR) or the effect of reducing the standard photoparoxysmal response range (SPR). Photosensitivity was detected using electroencephalography (EEG) measurements in subjects exposed to intermittent photostimulation (IPS). This EEG response is the photoparoxysmal response (PPR). For research purposes, it is desired that the PPR is triggered by the IPS, not the seizure. The SPR for each patient is the number of standard visual stimulus frequencies (in Hertz) to which the patient is sensitive, between the lower and upper thresholds. Test 14 frequencies (2-60Hz). The onset and duration of response (PPR inhib...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com