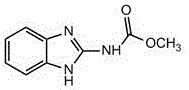
A kind of synthetic method of carbendazim hapten
A synthetic method and hapten technology, applied in the direction of chemical instruments and methods, material inspection products, instruments, etc., to achieve highly specific effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
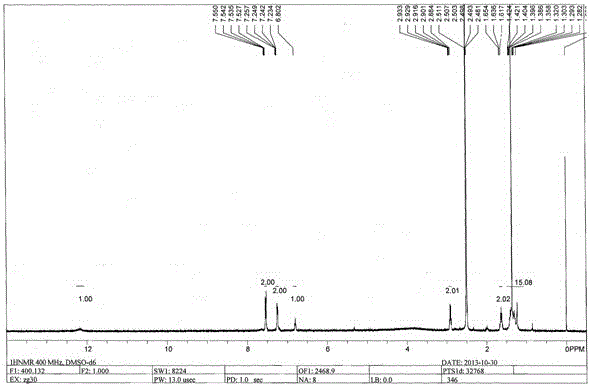
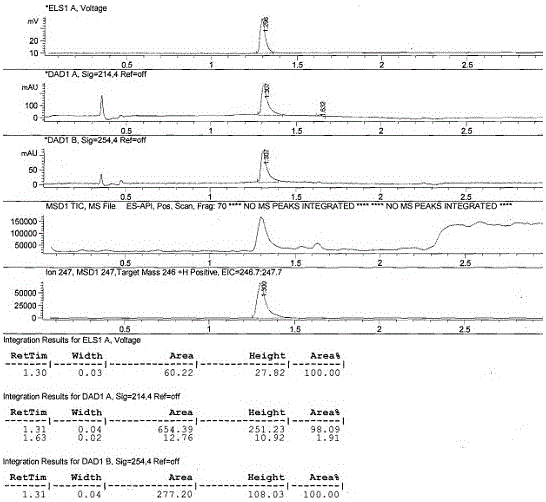
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 carbendazim hapten
[0027] (1) Synthesis of active ester 348: tert-butyloxycarbonyl (Boc)-protected aminocaproic acid, 1.155 g, 5 mmol, was dissolved in 20 mL of dichloromethane-tetrahydrofuran mixed solvent, the mixing volume ratio was 1:1, followed by Add the condensing agent 1-hydroxybenzotriazole monohydrate (HOBT) and 1-(3-dimethylaminopropyl)-3-ethyl carbon in an equimolar amount to the aminocaproic acid protected by tert-butyloxycarbonyl Diimine (EDCI), stirred at room temperature for 2h, set aside;
[0028] (2) Synthesis of precursor 346: 2-aminobenzimidazole (0.665 g) dissolved in a small amount of DMF was added to the active ester 348 solution prepared in step (1), and stirred at room temperature overnight; the reaction solution was rotary evaporated to remove dichloro Methane-tetrahydrofuran, the residue was dissolved in 20 mL of dichloromethane, washed three times with water, 20 mL each time; then washed three times with sat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com