Chiral compound comprising iminopyridyl oxazoline and preparation method thereof
A technology containing imine pyridine oxazoline and compound is applied in the field of synthesizing imine pyridine oxazoline-containing compound, can solve problems such as asymmetry, and achieve the effect of high chemical conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0065] Embodiment: Amine formula (3) is commercially available, and 2-bromo-6-acylpyridine formula (2) is according to literature (Ruifa Zong, Dong Wang, Richard Hammitt, and RandolphP.Thummel.J.Org.Chem. , 2006,71,167) prepared. Oxazoline ring formula (5) according to literature ((a) Bandyopadhyay, S.; Zhou, W.; Breslow, R.Org. Lett.2007, 9, 1009; (b) Levine, M.; Kenesky, C.S.; Zheng, S.; Quinn, J.; Breslow, R. Tetrahedron Lett. 2008, 49, 5746.) Preparation.
[0066] Preparation of 2-bromo-6-iminopyridine formula (4)
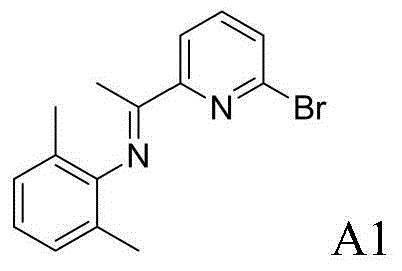
example A1
[0067] Example A1: Preparation of 2-bromo-6-iminopyridine A1
[0068]
[0069] 2,6-Dimethylaniline (2.9083g, 24mmol, 1.2equiv) and 2-bromo-6-acetylpyridine (4.0006g, 20mmol, 1.0equiv) were dissolved in 50mL of toluene, p-toluenesulfonic acid (0.0760g, 0.4mmol, 2mol%), reacted for 24h, and recrystallized from ethanol to obtain 4.7901g (15.8mmol, 79%) of 2-bromo-6-iminopyridine A1.
[0070] 1 H NMR (400MHz, CDCl 3 )δ8.33(d, J=7.7Hz, 1H), 7.64(t, J=7.7Hz, 1H), 7.56(d, J=7.7Hz, 1H), 7.06(d, J=7.5Hz, 2H) ,6.93(t,J=7.5Hz,1H),2.15(s,3H),2.01(s,6H). 13 C NMR (100MHz, CDCl 3 )δ166.15, 157.44, 148.44, 140.97, 138.74, 129.25, 127.95, 125.25, 123.26, 120.03, 17.90, 16.63.
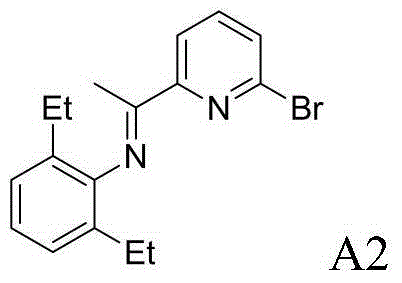
example A2
[0071] Example A2: Preparation of 2-bromo-6-iminopyridine A2
[0072]
[0073] 2,6-Diethylaniline (3.5815g, 24mmol, 1.2equiv) and 2-bromo-6-acetylpyridine (4.0006g, 20mmol, 1.0equiv) were dissolved in 50mL of toluene, p-toluenesulfonic acid (0.0760g, 0.4mmol, 2mol%) catalyzed, reacted for 24h, recrystallized from ethanol to obtain 5.5341g (16.7mmol, 84%) 2-bromo-6-iminopyridine A2.
[0074] 1 H NMR (400MHz, CDCl 3 )δ8.32(d, J=7.7Hz, 1H), 7.65(t, J=7.7Hz, 1H), 7.57(d, J=7.7Hz, 1H), 7.25–6.98(m, 3H), 2.34( m, 4H), 2.17(s, 3H), 1.12(t, J=7.5Hz, 6H). 13 C NMR (100MHz, CDCl 3 )δ165.90,157.46,147.47,140.99,138.75,131.05,129.21,125.99,123.55,119.99,24.57,16.96,13.70.calcd for m / z C 17 h 19 BrN 2 330.0732,found m / z330.0735.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com