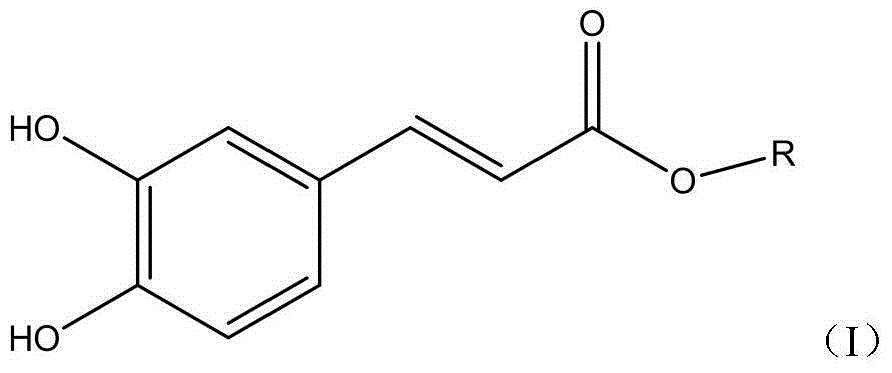
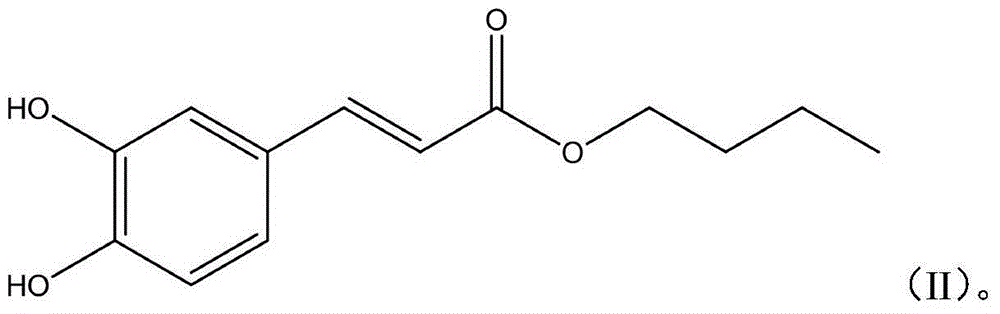
Applications of caffeic acid alkyl ester
A technology of alkyl esters and caffeic acid, applied in the preparation of broad-spectrum bacteriostatic agents, in the field of alkyl caffeic acid esters, can solve problems that have not been reported, and achieve the effect of inhibiting pathogenic bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The present embodiment adopts spectrophotometry to detect the caffeic acid alkyl ester (comprising methyl caffeate, ethyl caffeate, propyl caffeate, isopropyl caffeate, butyl caffeate, pentyl caffeate, Caffeic acid isoamyl ester, caffeic acid hexyl ester) to the inhibitory effect of Pseudomonas mulberry, specific method is as follows:
[0033] Pseudomonas syringae was shaken and cultivated in LB liquid medium at 150r / min and 30°C overnight to obtain a bacterial suspension.
[0034] Alkyl caffeate was dissolved in a co-solvent to prepare a 60 g / L solution, which was sterilized by filtration with a 0.22 μm microporous membrane.
[0035] Prepare LB liquid medium, put 2mL liquid medium in each test tube, filter and sterilize the caffeic acid alkyl ester with a 0.22 μm microporous membrane, and set different concentrations of the caffeic acid alkyl ester.
[0036] Each test tube was inoculated, and each bacterial strain and each caffeic acid alkyl ester were repeated three ...
Embodiment 2
[0041] The present embodiment adopts spectrophotometry to detect the caffeic acid alkyl ester (comprising methyl caffeate, ethyl caffeate, propyl caffeate, isopropyl caffeate, butyl caffeate, pentyl caffeate, Caffeic acid isoamyl ester, caffeic acid hexyl ester) to the inhibitory action of Pseudomonas mulberry, concrete method is as embodiment 1:
[0042] Pseudomonas syringae was shaken and cultivated in LB liquid medium at 150r / min and 30°C overnight to obtain a bacterial suspension.
[0043] Alkyl caffeate was dissolved in a co-solvent to prepare a 60 g / L solution, which was sterilized by filtration with a 0.22 μm microporous membrane.
[0044] Prepare LB liquid medium, put 2mL liquid medium in each test tube, filter and sterilize the caffeic acid alkyl ester with a 0.22 μm microporous membrane, and set different concentrations of the caffeic acid alkyl ester.
[0045] Each test tube was inoculated, and each bacterial strain and each caffeic acid alkyl ester were repeated t...
Embodiment 3
[0050] The present embodiment adopts spectrophotometry to detect the caffeic acid alkyl ester (comprising methyl caffeate, ethyl caffeate, propyl caffeate, isopropyl caffeate, butyl caffeate, pentyl caffeate) of optimal concentration range , caffeic acid isoamyl ester, hexyl caffeate) to the inhibition of Pseudomonas syringae, calculate the half inhibitory concentration of alkyl caffeate to Pseudomonas syringae, concrete method is as embodiment 1:
[0051] Pseudomonas syringae was shaken and cultivated in LB liquid medium at 150r / min and 30°C overnight to obtain a bacterial suspension.
[0052] Alkyl caffeate was dissolved in a co-solvent to prepare a 60 g / L solution, which was sterilized by filtration with a 0.22 μm microporous membrane.
[0053] Prepare LB liquid medium, put 2mL liquid medium in each test tube, filter and sterilize the caffeic acid alkyl ester with a 0.22 μm microporous membrane, and set different concentrations of the caffeic acid alkyl ester.
[0054] Eac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com