polyacrylate allophanate
A technology of allophanate and acrylate, applied in polyurea/polyurethane coatings, synthetic resin layered products, organic chemistry, etc., can solve problems such as over-sticking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
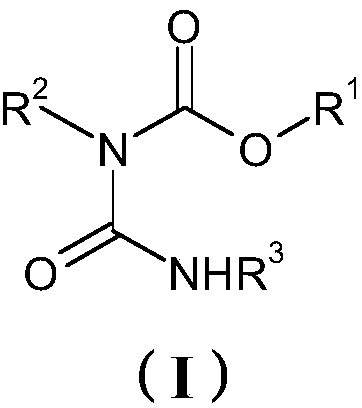
[0117] Embodiment 1: the preparation of the allophanate of formula (I)
[0118] In a fully stirred jacketed reactor introduce:
[0119] 425 g of HDI (2.53 mol), followed by 82 g of ethoxylated C 12 -C 18 Alcohol (0.23 mol) + 1.45 g of 1-butanol / 2-butanol mixture (75 / 25 in mass ratio m / m) and 5.85 g of catalyst KKAT XK-629 (at ambient temperature). The medium is heated to reach a temperature of 110° C. within 2 hours. The reaction medium is maintained at this temperature for about 1.5 hours.
[0120] The NCO content of the reaction medium was measured regularly by means of dibutylamine return dosing.
[0121] When the NCO content of the reaction medium corresponds to the desired theoretical content, the reaction is stopped by adding 0.066 g of p-toluenesulfonic acid.
[0122] After 15 minutes, the temperature of the reaction medium returned to ambient temperature.
[0123] The NCO content of the final reaction medium is 0.829 mol of NCO for 100 g.
[0124] Two successi...
Embodiment 2
[0129] Embodiment 2: Preparation according to the modified allophanate of the present invention
[0130] 80 g (0.205 mol) of (pentaerythritol) triacrylate (PETIA), 0.02 g of dibutyltin dilaurate (DBTL), 0.072 g of butyltin were introduced into a three-necked flask equipped with a cooling system, a mechanical stirrer and a nitrogen inlet. hydroxytoluene (BHT) and 100 g of dry toluene.
[0131] Then 61.4 g (0.185 mol) of the allophanate of the formula (I) of Example 1 were added dropwise with stirring, and the reaction medium was then heated to a temperature of 60°C.
[0132] The reaction was stopped after 7 hours when the NCO groups had reacted completely, and the reaction medium was returned to ambient temperature.
[0133] The solvent was then evaporated in vacuo.
Embodiment 6-8
[0142] Embodiment 6-8: by the product production coating of embodiment 2,3,4
[0143] The products of Examples 2, 3 and 4 were used to produce coatings which were crosslinkable under UV under the conditions shown in Table 3.
[0144] The urethane acrylate based formulation was adjusted to 50% dry extract with acetone and then 4% photoinitiator (Irgacure 500) was added.
[0145] 12 μm were applied on polycarbonate panels using a K-rod. After evaporating the solvent (30 minutes at 60° C. in an oven), the plates were kept under constant temperature and humidity conditions (50% RH, 23° C.) for 24 hours. The thickness of the coating is then 6 μm.
[0146] The plates were then crosslinked under UV (mercury lamp) under the conditions indicated in Table 3.
[0147] table 3
[0148]
[0149] Evaluation of the following properties was carried out 24 hours after crosslinking.
[0150] Gloss
[0151] Gloss was measured at an angle of 20° initially and after 50 reciprocations ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com