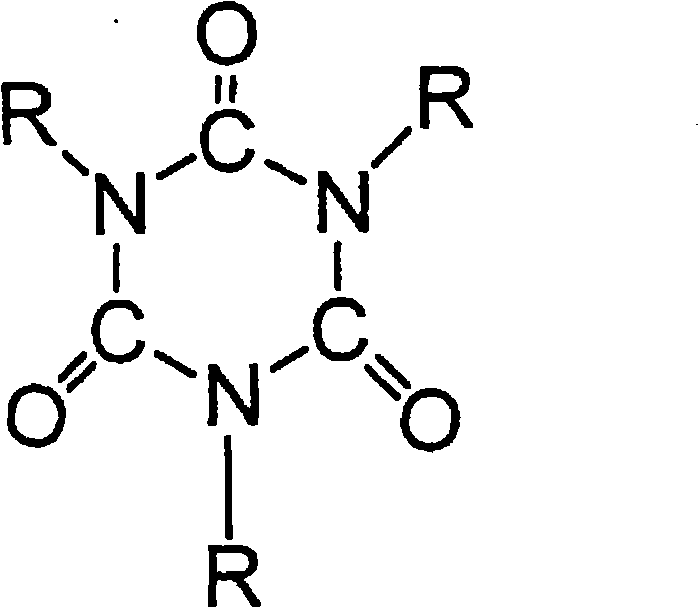
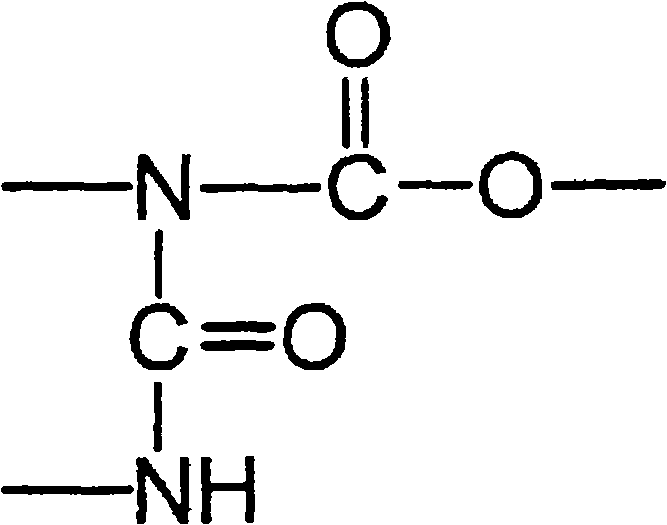
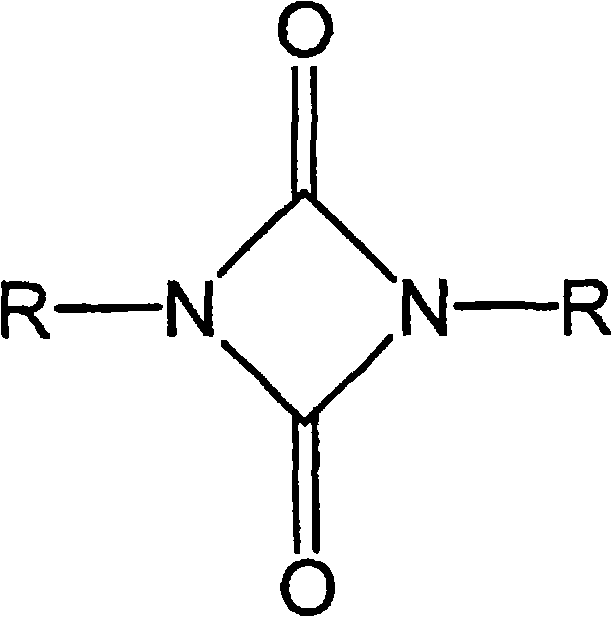
Highly crosslinkable low-viscosity polyisocyanate composition and coating composition containing same
A polyisocyanate and diisocyanate technology, applied in the direction of polyurea/polyurethane coatings, coatings, etc., can solve the problems of limited use, low crosslinking, and increased monomer concentration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] (Example 1) (manufacture of polyisocyanate composition)
[0132] Make the interior of the four-necked flask equipped with a stirrer, a thermometer, a reflux condenser, a nitrogen blowing tube, and a dropping funnel into a nitrogen atmosphere, and put in 600 parts of HDI and 0.6 parts of isobutanol, and keep the temperature in the reactor at 80 °C under stirring. 2 hours. After that, an isocyanurate catalyst tetramethylammonium caprate was added to carry out isocyanurate reaction, and when the conversion rate reached 20%, phosphoric acid was added to stop the reaction. The mass concentration of uretdione dimer increased in this reaction is below 1%. The reaction liquid was further kept at 160° C. for 1 hour. Polyisocyanate containing uretdione groups is produced by heating. The reaction solution was filtered after cooling, and then unreacted HDI was removed using a thin film evaporator. Table 1 shows the characteristics of the obtained polyisocyanate composition.
Embodiment 2
[0134] It carried out similarly to Example 1 except having set the conversion rate of the isocyanuration reaction to 13%. The results are shown in Table 1.
Embodiment 3
[0136] It carried out similarly to Example 1 except having set the conversion rate of the isocyanuration reaction to 8%. The results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com