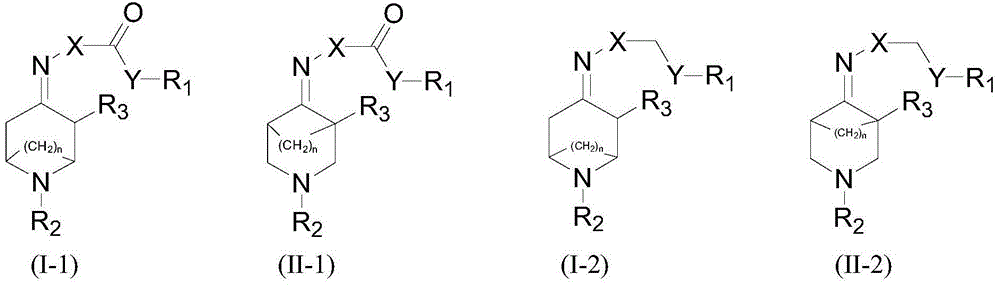
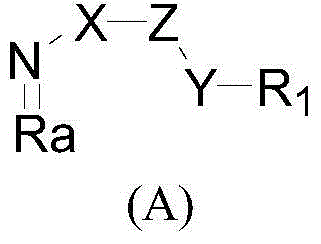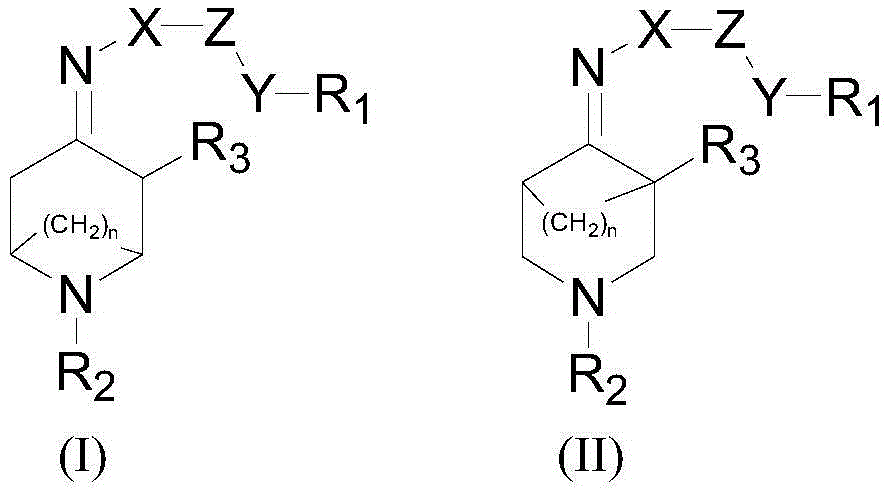Azabicyalo derivative as well as preparation and application thereof
An azabicycle, derivative technology, applied in the fields of botanical equipment and methods, applications, chemicals for biological control, etc., can solve problems such as environmental damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] The preparation methods of the compounds of the present invention are described in more detail below, but these specific methods do not constitute any limitation to the present invention. The compound of the present invention can also be conveniently prepared by optionally combining various synthetic methods described in the specification or known in the art. Such a combination can be easily performed by those skilled in the art to which the present invention belongs.
[0077]Usually, in the preparation process, each reaction is usually carried out in an inert solvent at room temperature to reflux temperature (such as 20°C-160°C, preferably 50°C-120°C). The reaction time is usually 0.1 hour to 60 hours, preferably 0.5 to 1 hour.
[0078] For the convenience of description, hereinafter, the compound represented by formula 1 is referred to as compound 1 for short, and the rest are deduced by analogy.
[0079] In a preferred example, compound (III) is taken as an example ...
preparation example 1
[0120] Intermediate Preparation Example 1: Synthesis of Tropinone Oxime
[0121]
[0122] 1.39g (10mmol) of tropinone, 1.05g (15mmol) of hydroxylamine hydrochloride and 1.60g (20mmol) of anhydrous acetic acid manganese were dissolved in 30ml of absolute ethanol solution, and the system was stirred and refluxed for 3 to 4 hours. Pinone reacts completely. Add 10% NaOH solution to adjust the pH value to 10-11. The solvent ethanol was distilled off to obtain the crude product, which was washed with water, extracted with ethyl acetate and anhydrous MgSO 4 After drying, the solvent was removed to obtain a pure product with a yield of 90%. 1 HNMR (400MHz,) δ3.28(d, J=11.7Hz, 2H), 2.98(d, J=15.5Hz, 1H), 2.59(d, J=14.7Hz, 1H), 2.36(s, 3H), 2.22(dd, J=15.4, 2.9Hz, 1H), 2.12(d, J=14.7Hz, 1H), 1.99(d, J=3.8Hz, 2H), 1.58(t, J=9.1Hz, 1), 1.47(t,J=9.0Hz,1H). 13 CNMR (101MHz, CDCl 3 )δ155.6,60.7,59.9,39.0,37.0,30.9,27.2,26.3.MS(GC-MS):m / z154(M + ), 137, 96, 82.
preparation example 2
[0124] 2.12-(allyloxy)isoindoline-1,3-dione
[0125] Dissolve 8.1g (50mmol) N-hydroxyphthalimide and 6.9g (50mmol) anhydrous potassium carbonate in 100mL DMF, slowly add 6g (50mmol) allyl bromide dropwise, and stir at room temperature. According to TLC tracking, the reaction ended after 24 hours. The reaction solution was poured into ice water, and a white solid was precipitated, filtered, washed with deionized water, and dried to obtain 29.1 g of a white solid intermediate with a yield of 90%. Melting point: 55-57°C; 1 HNMR (400MHz, CDCl 3 )δ7.84(s,2H),7.78(s,2H),6.21–6.08(m,1H),5.44–5.32(m,2H),4.75–4.69(m,2H). 13 CNMR (101MHz, CDCl 3 )δ163.7, 134.4, 131.2, 128.8, 123.5, 122.5, 78.8.
[0126] 2.2O-allyl hydroxylamine hydrochloride
[0127] Add 9.1g (45mmol) of 2-(allyloxy)isoindoline-1,3-dione and 2.81g (45mmol) of 80% hydrazine hydrate into 100mL of absolute ethanol, heat and reflux for 2 hours, and then Cool down to room temperature and continue to stir the reaction f...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap