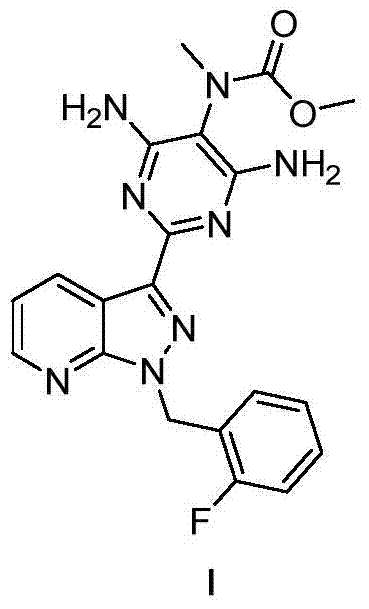
A kind of compound and its application in preparing riociguat
A compound and mixture technology, applied in the field of drug synthesis, can solve problems such as side reactions, impurities, and product purification difficulties, and achieve significant economic significance, mild reaction conditions, and high product purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
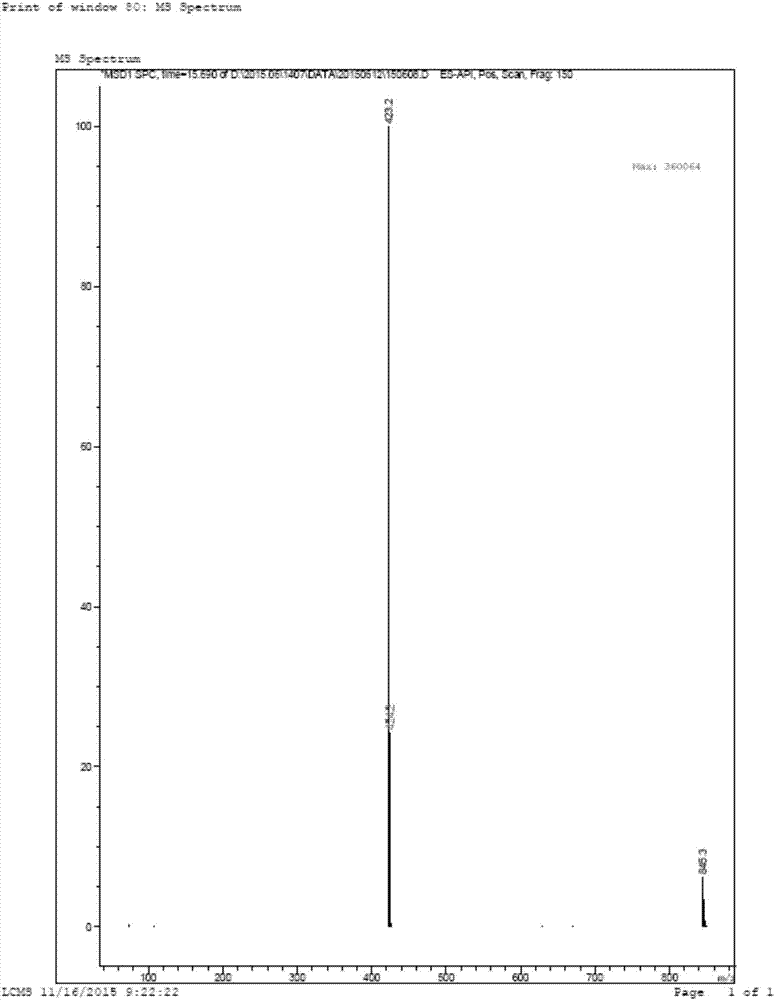
[0071] 5-bromo-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidine-4,6-diamine (compound III, LG is bromo ) preparation
[0072]
[0073] Add 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine (compound IV) (2.7g, 10mmol) and sodium methoxide (0.7g , 15mmol) and toluene 35mL, 2-bromomalononitrile (1.43g, 10mmol) was added under stirring at room temperature, replaced with nitrogen three times, heated to reflux for 5 hours, then cooled to room temperature, solids were precipitated, filtered, and the filter cake was used Cold toluene (20 mL) washed. Obtained 3.39g of yellow solid, that is, 5-bromo-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidine-4,6-di Amine, yield 82%.
[0074] 1 H NMR (DMSO-d6): δ=8.92 (dd, 1H), 8.76 (dd, 1H), 7.63 (m, 2H), 7.54 (m, 2H), 7.10 (m, 1H), 6.76 (brs, 4H ), 5.68(s, 2H).
Embodiment 2
[0076] 5-methylsulfonate-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidine-4,6-diamine (compound III, LG is the preparation of mesylate)
[0077]
[0078]Add 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine (IV) (2.7g, 10mmol), potassium tert-butoxide (1.68 g, 15mmol) and 35mL N,N-dimethylformamide, add 2-methanesulfonyl malononitrile (1.6g, 10mmol) under stirring at room temperature, replace with nitrogen three times, heat to 80°C, and stir for 5 hours , cooled to room temperature, a solid precipitated, filtered, and the filter cake was washed with cold toluene (20 mL). 3.65g of yellow solid was obtained, which was 5-mesylate-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidine-4, 6-diamine, yield 85%.
[0079] 1 H NMR (DMSO-d6): δ=8.92 (dd, 1H), 8.72 (dd, 1H), 7.64 (m, 2H), 7.54 (m, 2H), 7.10 (m, 1H), 6.94 (brs, 4H ), 5.68 (s, 2H), 3.59 (s, 3H).
Embodiment 3
[0081] 5-iodo-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidine-4,6-diamine (compound III, LG is iodine ) preparation
[0082]
[0083] Add 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine (IV) (2.7g, 10mmol) and potassium carbonate (0.7g, 15mmol) and 30mL dimethyl sulfoxide, added 2-iodomalononitrile (1.91g, 10mmol) under stirring at room temperature, replaced three times with nitrogen, stirred at room temperature for 48 hours, added 30mL of water, solids were precipitated, and the filter cake was used Washing with toluene (20 mL) gave 3.13 g of a yellow solid, namely 5-iodo-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidine -4,6-diamine, the yield is 68%.
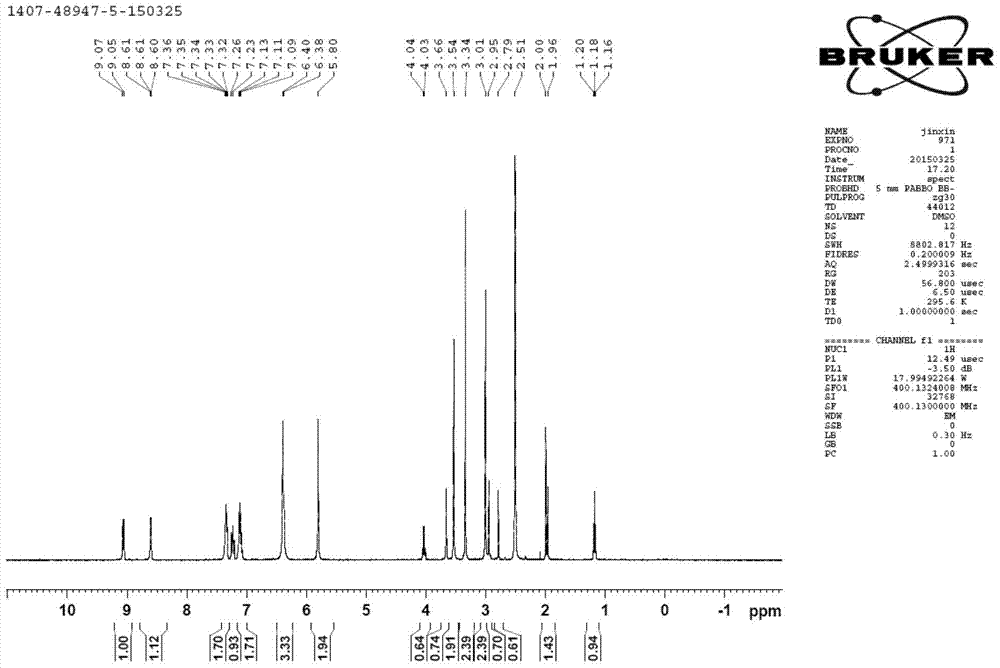
[0084] 1 H NMR (DMSO-d6): δ=8.92 (dd, 1H), 8.76 (dd, 1H), 7.63 (m, 2H), 7.54 (m, 2H), 7.10 (m, 1H), 6.76 (brs, 4H ), 5.72(s, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com