Method for synthesizing famciclovir intermediate
A technology of famciclovir intermediates and synthetic methods, which is applied in the field of synthesis of famciclovir intermediates, can solve the problems of uneconomical, cumbersome operations, and large amounts of reagents, and achieve the effects of reduced production costs and reduced amounts of reducing agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
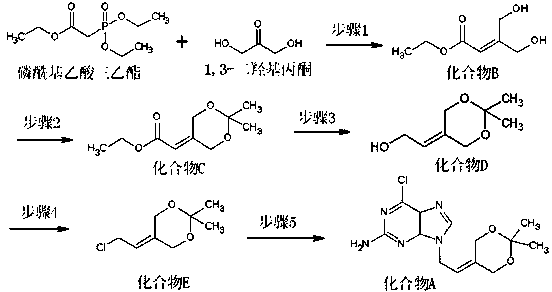
[0024] Add 40 grams of triethyl phosphoroacetate, 16 grams of 1,3-dihydroxyacetone, 200 ml of water, and 21.8 grams of sodium carbonate into the reaction bottle, and react at 20-30°C for 4 hours. The aqueous layer was saturated with sodium chloride, and the aqueous layer was extracted three times with 500 ml of tetrahydrofuran. The organic layers were combined, dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain 28.1 g of compound B as a light yellow oil.
Embodiment 2
[0026] In the reaction flask, add 25.4 g of compound B, dissolve in 300 ml of acetone, 1 g of p-toluenesulfonic acid, react at 40-50 ° C for 8 hours, concentrate the solvent, add 200 ml of dichloromethane to dissolve the residue, and wash with 100 ml of saturated aqueous sodium bicarbonate The organic layer was separated, dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain 27.6 g of compound C as a pale yellow oil.
Embodiment 3
[0028] In the reaction flask, add 25.4 grams of compound B, dissolve in 200ml of acetone, then add 38.9ml of 2,2-dimethoxypropane, 0.3g of p-toluenesulfonic acid, react at 30-40°C for 8 hours, concentrate the solvent, and add the remainder to Dissolved in 200 ml of dichloromethane, washed the organic layer with 100 ml of saturated aqueous sodium bicarbonate solution, separated the organic layer, dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain 30.2 g of compound C as a light yellow oil.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com