A kind of method for preparing riociguat
A riociguat and intermediate technology, which is applied in the field of pharmaceutical synthesis, can solve the problems of easy occurrence of side reactions, easy generation of impurities, difficult product purification, etc., and achieves the effects of high yield and purity, easy purification and production cost saving.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] For the preparation method of the intermediate V in step (1), those skilled in the art can make various choices, and routinely select or determine through experiments the reagents and reaction conditions used in each method.
[0055] The carbamate reaction of step (4) is to introduce a formate group to the methylamino group at position 5 on the pyrimidine ring of the intermediate II. Those skilled in the art can make a variety of choices for this carbamate reaction, and the reagents and reaction conditions used for each mode are selected routinely or determined by experiment.
[0056] The yield and purity of the riociguat obtained by the synthesis process designed in the present invention are very high, the occurrence of side reactions is effectively reduced, the quality of the drug is improved, and the production cost is reduced.
Embodiment 1
[0065] Synthesis of 2-Methylaminomalononitrile VI
[0066]
[0067] Add bromomalononitrile (7.25g, 50mmol) and acetonitrile (100mL) into a 250mL three-necked flask, cool to 0°C, add potassium carbonate (13.8g, 100mmol), add methylamine hydrochloride (10.2g, 75mmol), stirred for 2 hours and filtered to remove the solid, the mother liquor was concentrated, dissolved in ethyl acetate (100mL), washed twice with saturated aqueous sodium bicarbonate (100mL×2), the organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated 3.8 g of brown solid was obtained, which was 2-methylaminomalononitrile, which could be directly used in the next reaction.
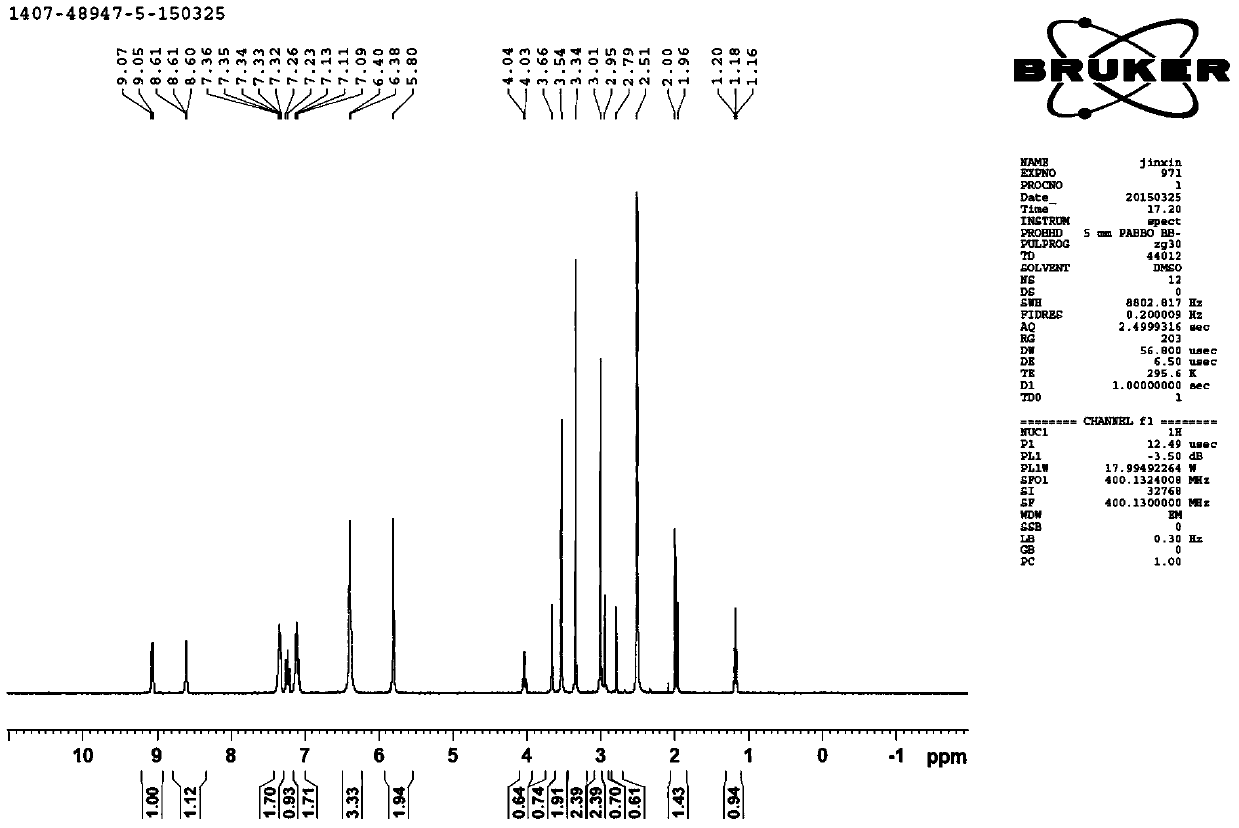
[0068] 1-H-NMR: (DMSO-d6,400M),5.0(S,1H),3.7(s,1H),3.0(s,3H)
Embodiment 2
[0070] Synthesis of 2-benzyl(methyl)aminomalononitrile V
[0071]
[0072] In a 250mL three-necked flask, the 2-methylaminomalononitrile (1.9g, 20mmol) prepared in Example 1 was added in acetonitrile (20mL), cooled to 0°C, then potassium carbonate (5.52g, 40mmol) was added, and Add benzyl chloride (2.77, 22mmol), stir for 2 hours and filter to remove the solid, concentrate the mother liquor, dissolve with ethyl acetate (100ml), wash twice with saturated aqueous sodium bicarbonate (100mL×2), and wash the organic phase with anhydrous sulfuric acid Sodium-dried, filtered, and concentrated to obtain 3.5 g of a brown solid, which is 2-benzyl (methyl) aminomalononitrile, which can be directly used in the next reaction.
[0073] 1-H-NMR: (DMSO-d6,400M),7.1-7.3(m,5H),5.0(s,2H),4.0(s,1H),3.2(s,3H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com