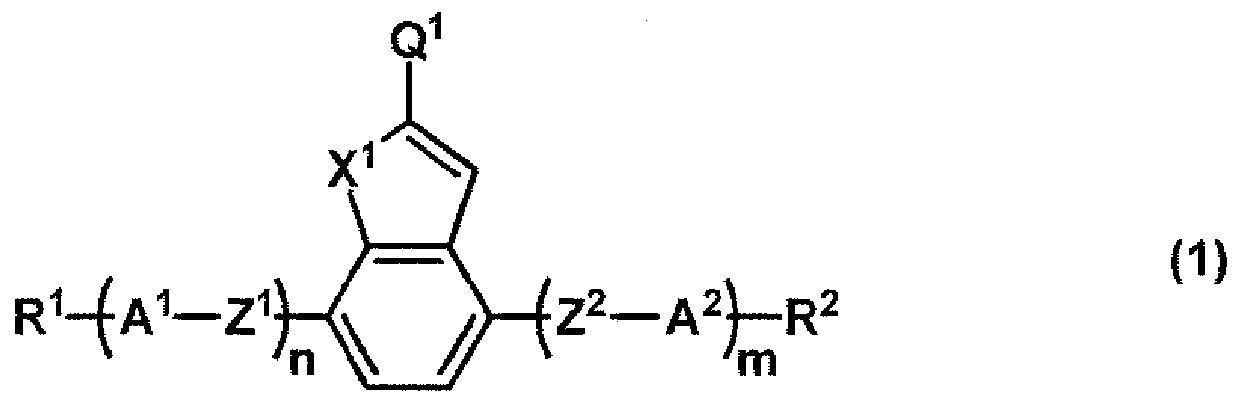
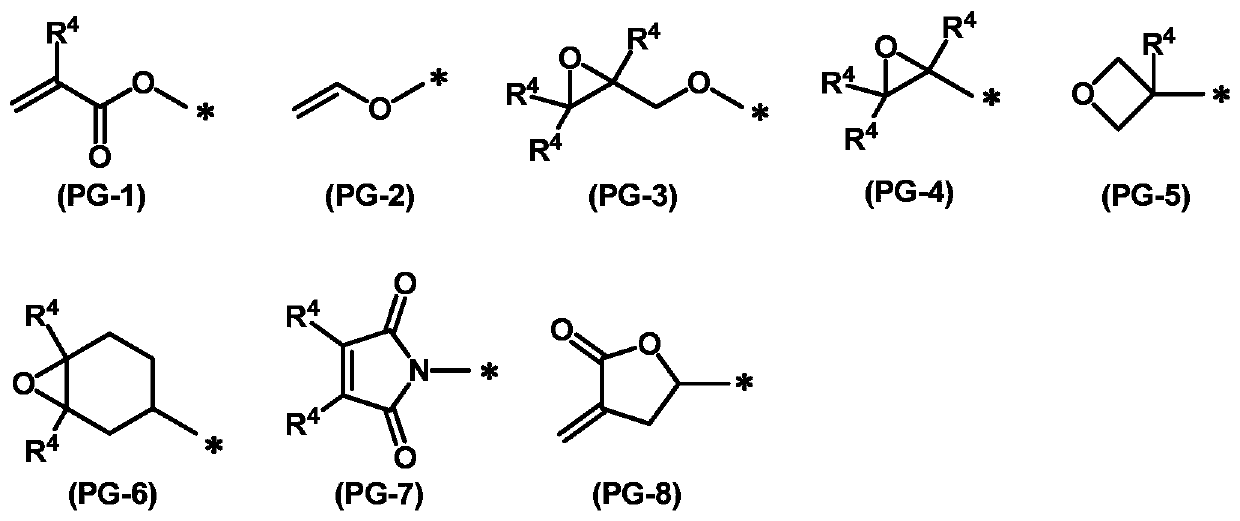
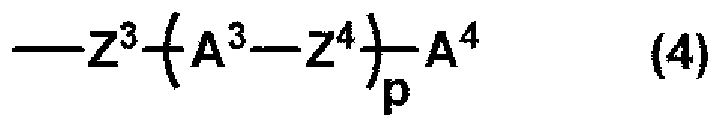Liquid crystal compound, liquid crystal composition, polymer and application thereof
A technology for liquid crystal compositions and compounds, applied in liquid crystal materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of low refractive index anisotropy, thicker film thickness, and long synthesis path.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0302] [Example 1] The compound (1-1-2-1) shown below was synthesized in the following manner.
[0303] [chem 38]
[0304]
[0305] Compound (ex-1) was synthesized according to "Heterocyclic communications" 8, 2, 135 (2002).
[0306] 16.0 g of compound (ex-1) was added to 160 mL of ethanol, and stirred at 10° C. or lower under a nitrogen atmosphere. 5.3 g of sodium borohydride was added thereto. Then, the mixture was stirred at below 10°C for 3 hours. 160 mL of 1N hydrochloric acid water was added to the reaction liquid, and then 160 mL of water was added. The precipitate was separated by filtration and washed with a large amount of water to obtain 15.5 g of compound (ex-2).
[0307] 5.0 g of compound (ex-2), 9.3 g of 4-pentylcyclohexanecarboxylic acid, and 1.1 g of 4-dimethylaminopyridine (4-dimethylaminopyridine, DMAP) were added to 90 ml of dichloromethane , stirring while cooling under a nitrogen atmosphere. 20 mL of a dichloromethane solution of 9.7 g of 1,3-dicy...
Embodiment 2
[0312] Compound (1-1-19-1) shown below was synthesized in the following manner.
[0313] [chem 39]
[0314]
[0315] Compound (ex-3) was synthesized according to "Heterocyclic communications." 8, 2, 135 (2002).
[0316] 5.0 g of compound (ex-3), 2.0 g of phenol, and 0.5 g of DMAP were added to 50 mL of dichloromethane, and stirred while cooling under a nitrogen atmosphere. 10 mL of a dichloromethane solution of 4.5 g of DCC was added dropwise thereto. After the dropwise addition, it was stirred at room temperature for 16 hours. The deposited precipitate was separated by filtration, and the organic layer was washed with water and dried over anhydrous magnesium sulfate. Dichloromethane was distilled off under reduced pressure, the residue was purified by column chromatography (silica gel, eluent: toluene), and recrystallized in methanol to obtain 4.8 g of the compound (ex- 4).
[0317] 4.8 g of compound (ex-4) was added to 150 mL of acetonitrile (MeCN), and stirred at ro...
Embodiment 3
[0324] Compound (1-1-7-1) shown below was synthesized in the following manner.
[0325] [chemical 40]
[0326]
[0327]Compound (ex-7) was synthesized according to "Heterocyclic communications." 8, 2, 135 (2002).
[0328] Add 5.0g of compound (ex-7), 3.2g of benzyl mercaptan, and 2.9g of potassium hydroxide to 50ml of N,N-dimethylformamide (dimethylformamide, DMF), under nitrogen atmosphere , 100 degreeC, heating and stirring for 4 hours. Water was poured into the reaction solution, and the precipitate was separated by filtration and washed with water and methanol. The obtained crystals were recrystallized from a mixed solution of toluene and methanol to obtain 3.5 g of compound (ex-8).
[0329] 3.0 g of compound (ex-8) was added to 90 mL of acetonitrile (MeCN), and stirred at room temperature under a nitrogen atmosphere. 18 mL of an aqueous solution of 12.8 g of CAN was added dropwise thereto. After the dropwise addition, the mixture was stirred at room temperature fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com