A pathogenic mutation of hereditary central halo retinopathy and its detection reagent
A retinopathy and hereditary technology, applied in the field of the pathogenic mutation of hereditary central halo retinopathy and its detection reagent, can solve the problems of unreported, cone and rod dystrophy, cone cell degeneration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] A five-generation family with central areolar choroidal dystrophy (CACD) was tested for GUCA1A mutations.
[0050] experimental method:
[0051] 1. Collection of clinical resources of the family and establishment of a genetic resource bank:
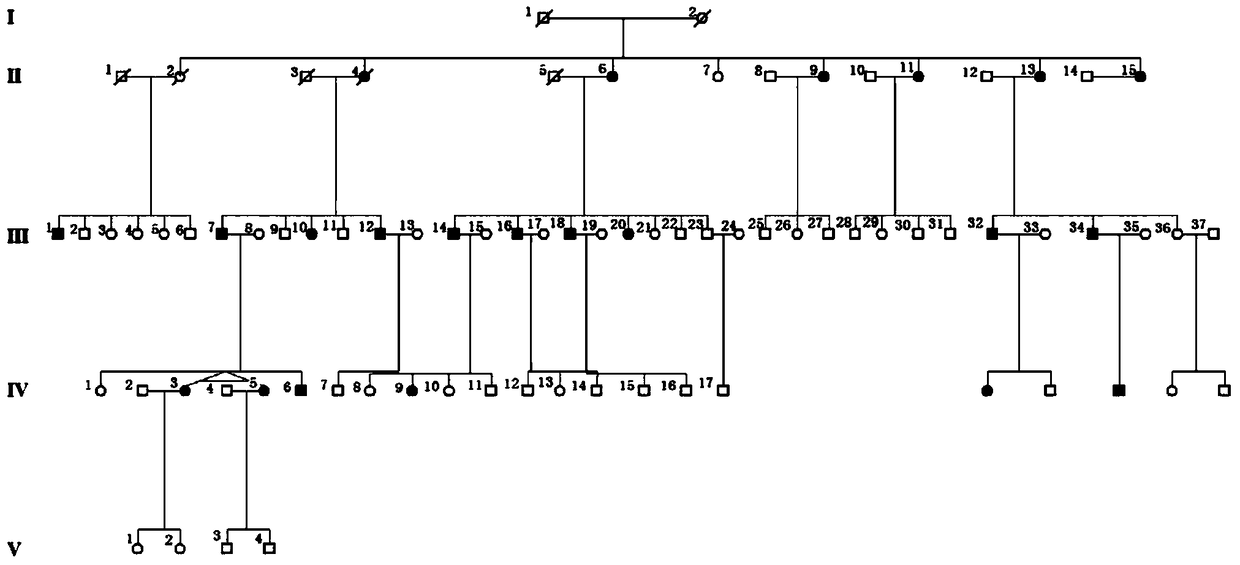
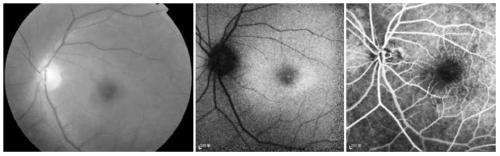
[0052] The clinical data and blood samples of each member of the family were collected, see the family diagram figure 1 . Clinical data mainly include personal medical history, family history, best corrected visual acuities (BCVAs), slit lamp examination, fundus photography, color vision examination, visual field examination (Humphrey perimetry), visual evoked potential detection (visual-evokedpotentials); VEP), full field electrophysiology (electroretinography; ERG), fundus fluoresceinangiography (FFA) and optical coherence tomography (optical coherencetomography; OCT), etc. The blood genomic DNA of each family member was extracted with a blood genomic DNA extraction kit (Qiagen, Hilden, Germany).
[0053] 2. Discover the path...
Embodiment 2
[0083] A functional study was conducted on the pathogenic mutation GUCA1A p.Arg120Leu detected in Example 1.
[0084] experimental method:
[0085] 1. Conservative analysis:
[0086] The NCBI HomoloGene database (http: / / www.ncbi.nlm.nih.gov / homologene) was used to evaluate and predict the conservation of the screened mutations in multiple species.
[0087] 2. Research on protein crystal structure changes:
[0088] SWISS MODEL (http: / / swissmodel.expasy.org / ) prediction software was used to predict the structure of GUCA1A wild-type protein and the mutant protein carrying the p.Arg120Leu mutation, and evaluate the protein structure changes caused by the mutation.
[0089] 3. Zebrafish experiment:
[0090] Synthesize the full-length cDNA of wild type and mutant human GUCA1A gene respectively and clone it into pxT7 vector to construct pxT7 tagged GUCA1A WT and GUCA1A MU Plasmids from which to synthesize the corresponding GUCA1A mRNA, including GUCA1A WT and GUCA1A MU mRNA. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com