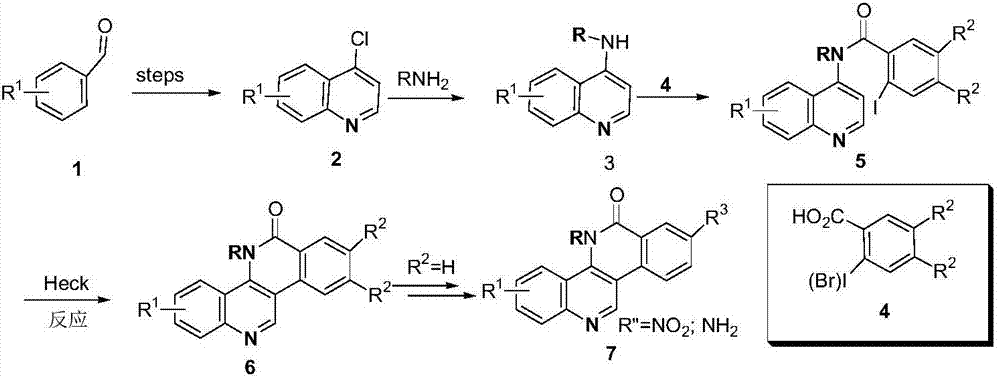
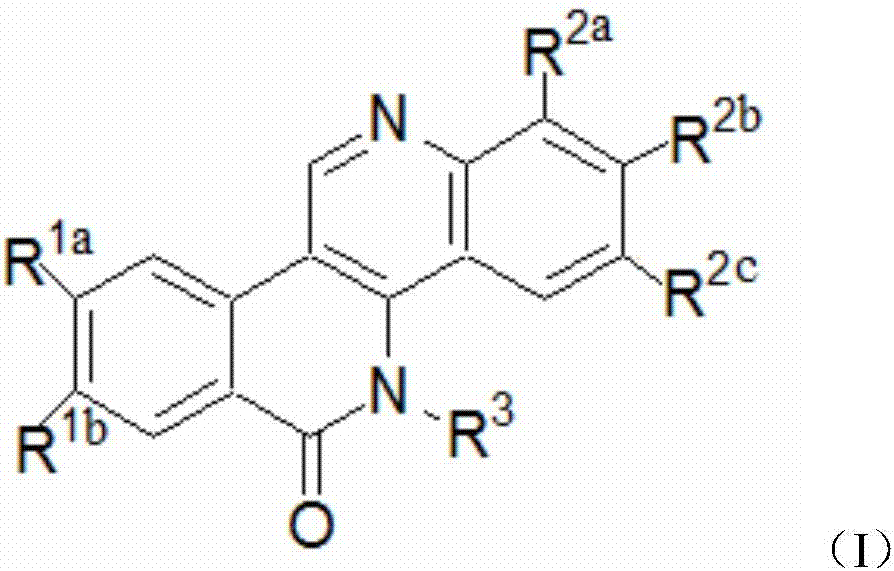
Dibenzonaphthyridinone compounds and their preparation methods and applications
A technology of benzonaphthyridone and compounds, which is applied in the field of anti-tumor compounds, and can solve the problems of not being easy to obtain and cumbersome steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
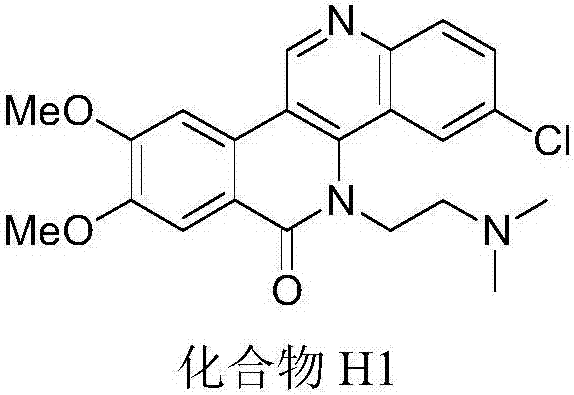
[0033] Preparation Example 1: Preparation of compound H1: 5H-8,9-dimethoxy-5-(2-N,N-dimethylaminoethyl)-3-chlorodibenzo[c,h][1,6]-naphthyridin-6-one
[0034]
[0035] (1) Add 3,4-dimethoxyphenylacetic acid (1.96g, 10mmol), absolute ethanol (5mL), cyclohexane (5mL) and concentrated sulfuric acid (1mL) to a 50mL round bottom flask under stirring. After installing the water trap and straight condenser, reflux at 100℃ until there is no more water in the water trap; after the reaction is completed, remove the cyclohexane and excess ethanol under reduced pressure, and add ethyl acetate to the flask Ester (30 mL), the mixture was washed twice with ice water, 30 mL each time, and then washed twice with saturated sodium bicarbonate solution, 30 mL each time, and extracted with organic solvent three times. Combine the organic layers and dry the organic layers with anhydrous magnesium sulfate , Filter, remove the solvent under reduced pressure, use silica gel column chromatography (eluent: ...
preparation Embodiment 2
[0043] Preparation Example 2: Preparation of Compound H2 5H-8,9-dimethoxy-5-(2-N,N-dimethylaminoethyl)-1-chlorodibenzo[c,h][1,6]-naphthyridin-6-one
[0044]
[0045] O-Chloroaniline was used instead of p-chloroaniline, and other preparation methods were the same as those in Preparation Example 1, to obtain the target compound H2 in white powder form.
[0046] Compound H2: yield 36%, m.p. 222~223°C; 1 H NMR(500MHz, CDCl 3 )δ: 2.26(s,6H,N(CH 3 ) 2 ),2.94(t,J=7.10Hz,2H,CH 2 ),4.05(s,3H,OCH 3 ),4.11(s,3H,OCH 3 ), 4.67(t,J=7.10Hz,2H,CH 2 ),7.49(dd,J=8.70Hz,7.45Hz,1H,ArH),7.69(s,1H,ArH),7.85(dd,J=7.45Hz,0.95Hz,1H,ArH),7.89(s,1H) ,ArH), 8.41(d,J=8.70Hz,1H,ArH),9.65(s,1H,CH=N); 13 C NMR(125MHz, CDCl 3 )δ: 45.89,49.47,56.38,56.43,57.77,102.16,108.87,112.63,119.91,120.13,120.50,123.78,125.55,127.08,129.52,134.52,141.26,144.89,146.09,150.91,154.42,163.89; HRMS(ESI ): m / z calculated value [M+H] + 412.1428(C 22 H 22 ClN 3 O 3 ), the experimental value is 412.1443.
preparation Embodiment 3
[0047] Preparation Example 3: Preparation of Compound H3 5H-8,9-dimethoxy-5-(2-N,N-dimethylaminoethyl)-2-chlorodibenzo[c,h][1,6]-naphthyridin-6-one
[0048]
[0049] Meta-chloroaniline was used instead of p-chloroaniline, and the other preparation methods were the same as those in Preparation Example 1, to obtain the target compound H3 in white powder form.
[0050] Compound H3: yield 14%, m.p.235~236℃; 1 H NMR(500MHz, CDCl 3 )δ: 2.31(s,6H,N(CH 3 ) 2 ),2.99(t,J=7.25Hz,2H,CH 2 ),4.03(s,3H,OCH 3 ),4.10(s,3H,OCH 3 ),4.61(t,J=7.25Hz,2H,CH 2 ), 7.52(dd,J=9.25Hz,2.30Hz,1H,ArH),7.63(s,1H,ArH),7.84(s,1H,ArH),8.10(d,J=2.30Hz,1H,ArH) ,8.45(d,J=9.25Hz,1H,ArH),9.48(s,1H,CH=N); 13 CNMR(125MHz, CDCl 3 )δ: 45.87,49.01,56.38,56.43,57.68,102.02,108.73,111.91,117.32,119.53,126.15,126.75,127.27,129.34,135.06,140.78,146.71,149.32,150.71,154.35,163.59; HRMS(ESI): m / z calculated value [M+H] + 412.1428(C 22 H 22 ClN 3 O 3 ), the experimental value is 412.1434.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com