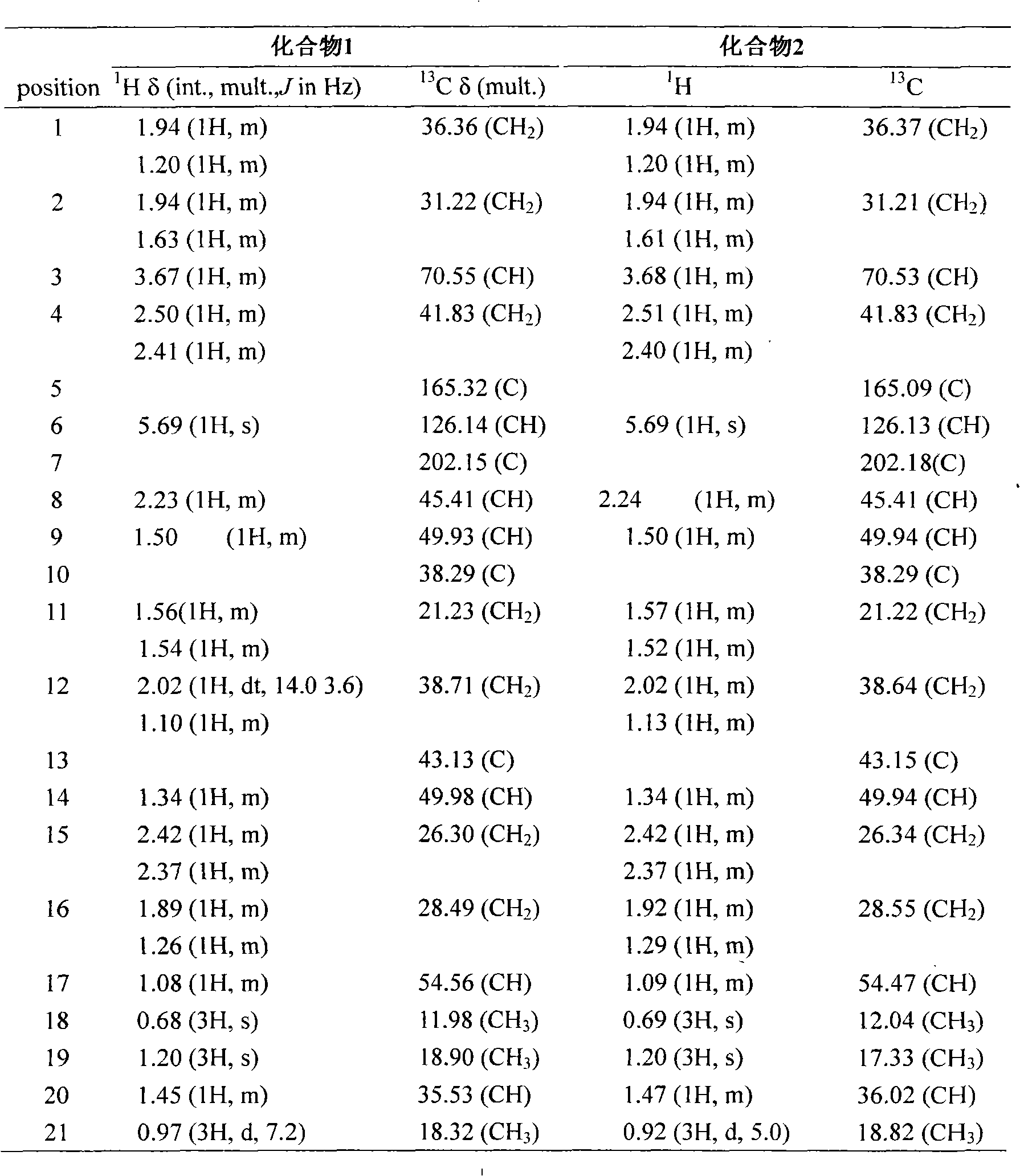
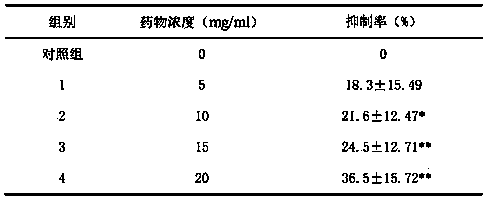
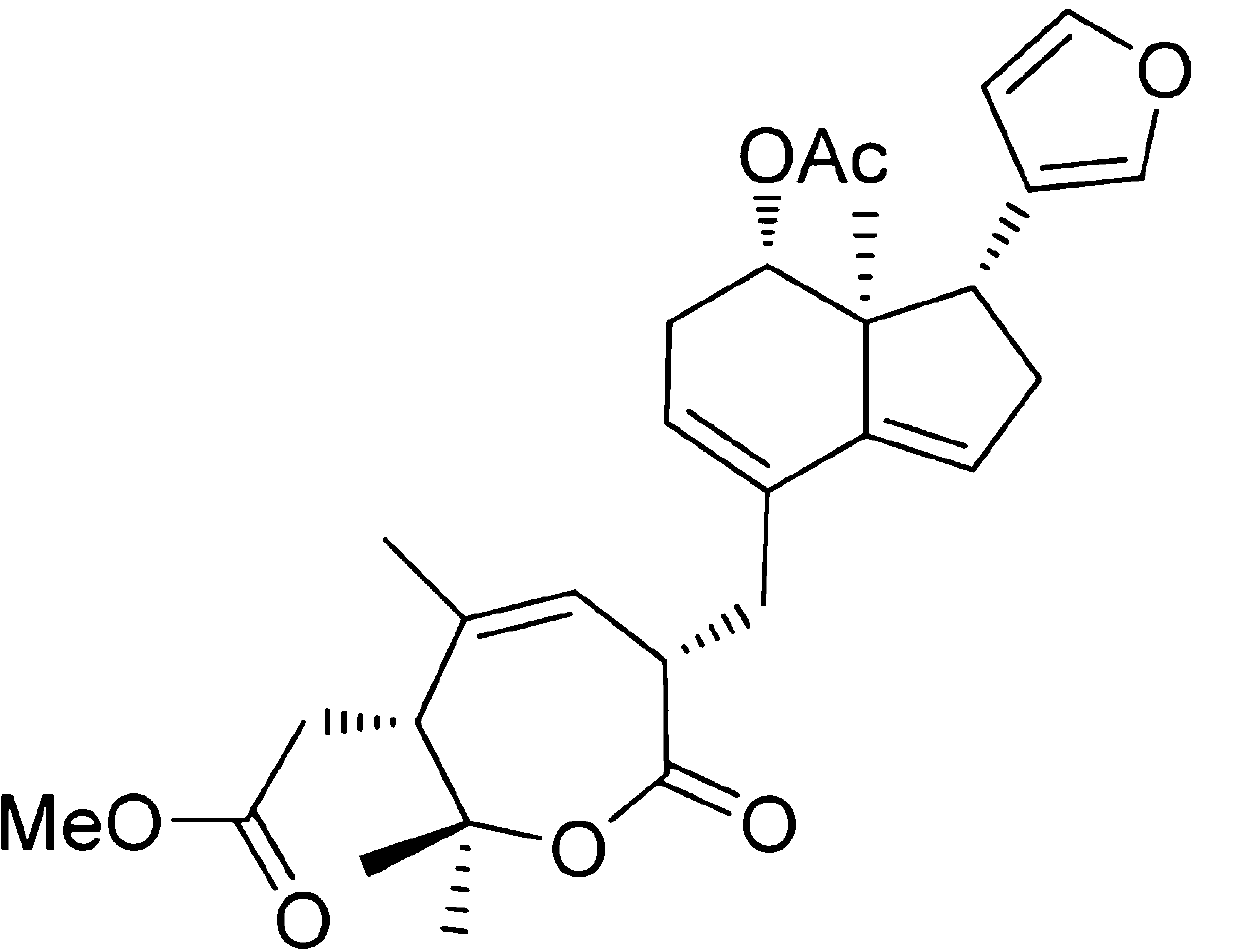
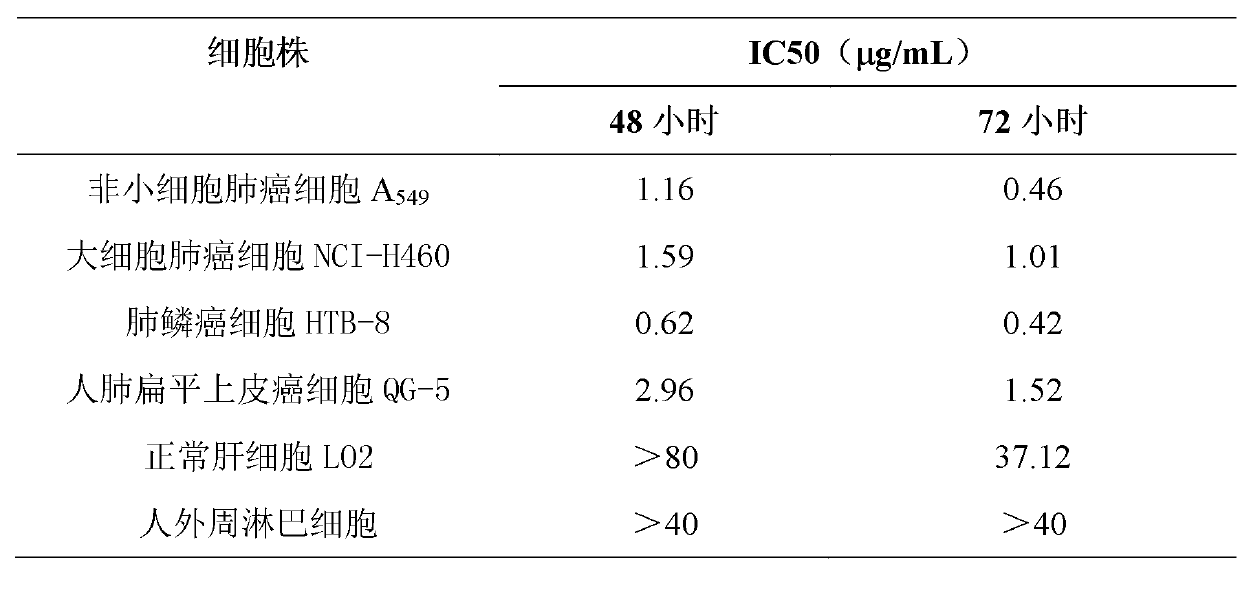
Patents
Literature
58 results about "Nci h460" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
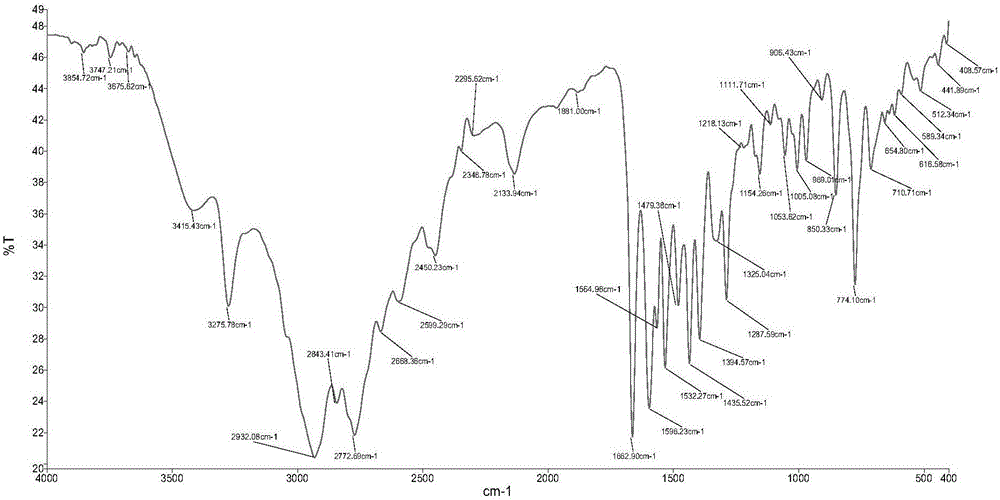
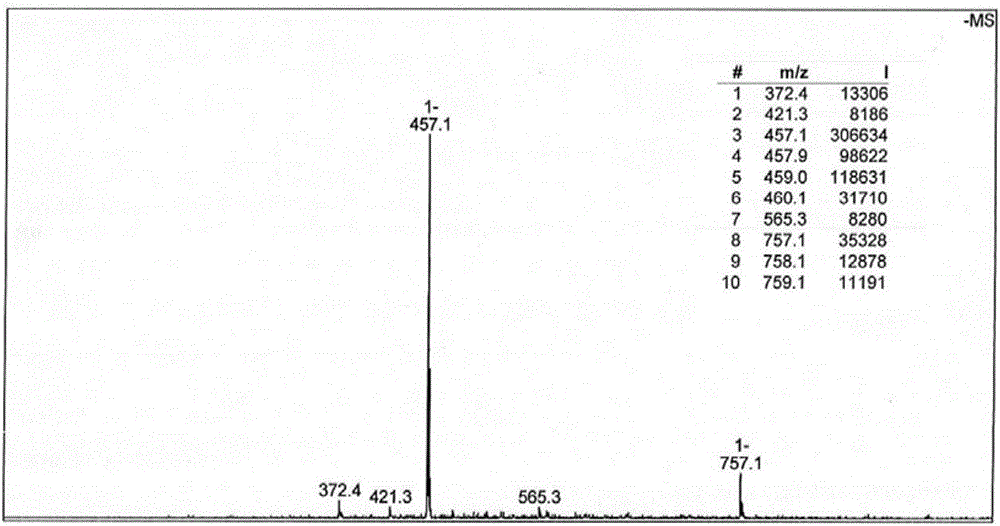
Synthesis method and application of platinum (II) complex of 6-amino oxoisoaporphine
InactiveCN103524564ASignificant in vitro antitumor activityGood potential medicinal valueOrganic active ingredientsGroup 8/9/10/18 element organic compoundsChemical structureHuman tumor
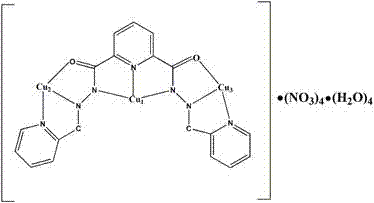
The invention discloses a new platinum (II) complex of 6-amino oxoisoaporphine, namely monochloro.dimethyl sulfoxide.6-amino oxoisoaporphine platinum (II), and a synthesis method and an application thereof. The complex is synthesized by the steps of dissolving 6-amino oxoisoaporphine and dichloro.di(dimethyl sulfoxide) platinum (II) in a polar solvent, and heating or carrying out a reflux reaction to obtain the target product; specifically the complex can be synthesized by a solution method, and can also be synthesized by a solvothermal method. Through investigation of proliferation inhibitory activity of the complex against HepG2, BEL-7404 and NCI-H460 and other human tumor cell strains, the complex is found to have significant in-vitro antitumor activity against the 3 kinds of tumor strains and have relatively good potential medicinal value, and is expected to be used in preparation of various antitumor medicines. The complex has the chemical structure represented by the following formula as described in the specification.
Owner:GUANGXI NORMAL UNIV
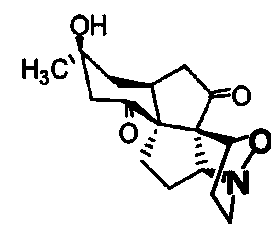
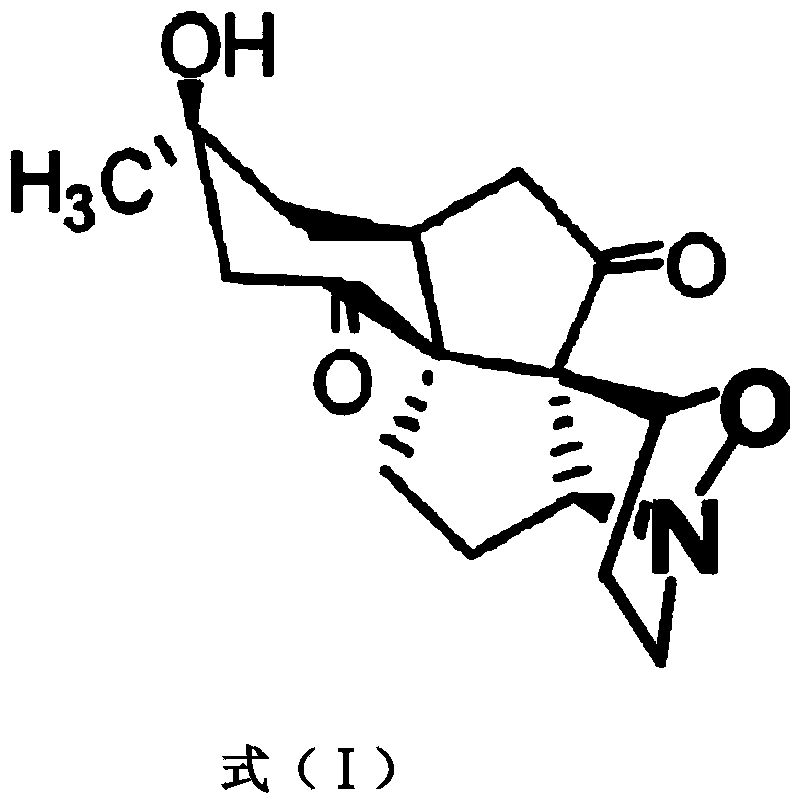
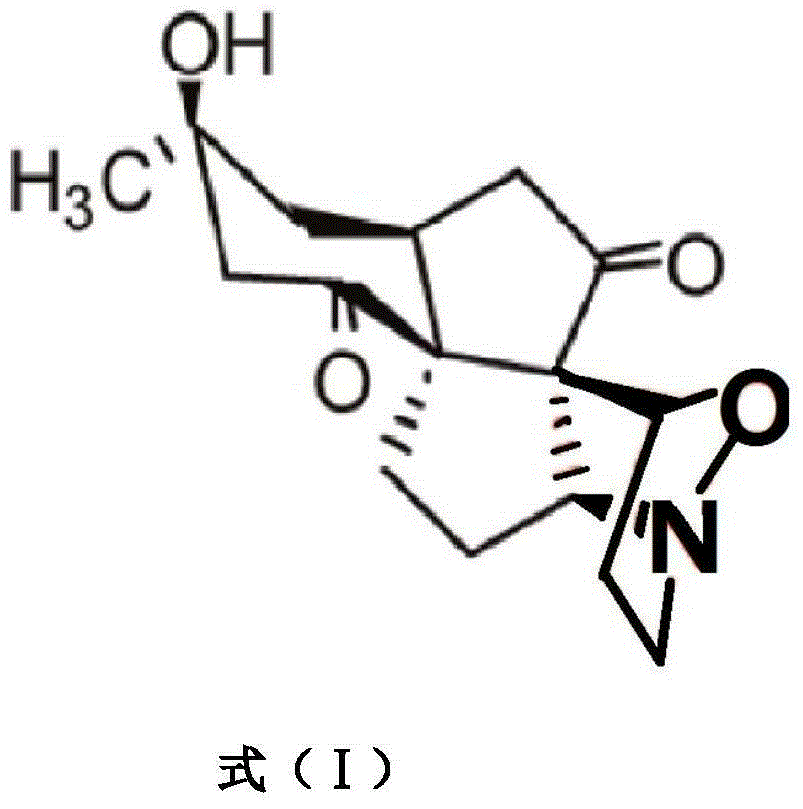
Platinum (II) metal complex for specifically inhibiting proliferation of lung carcinoma cell, and synthesis method and application thereof
ActiveCN106496280APrevent proliferationGood potential medicinal valueOrganic active ingredientsPlatinum organic compoundsIc50 valuesPlatinum
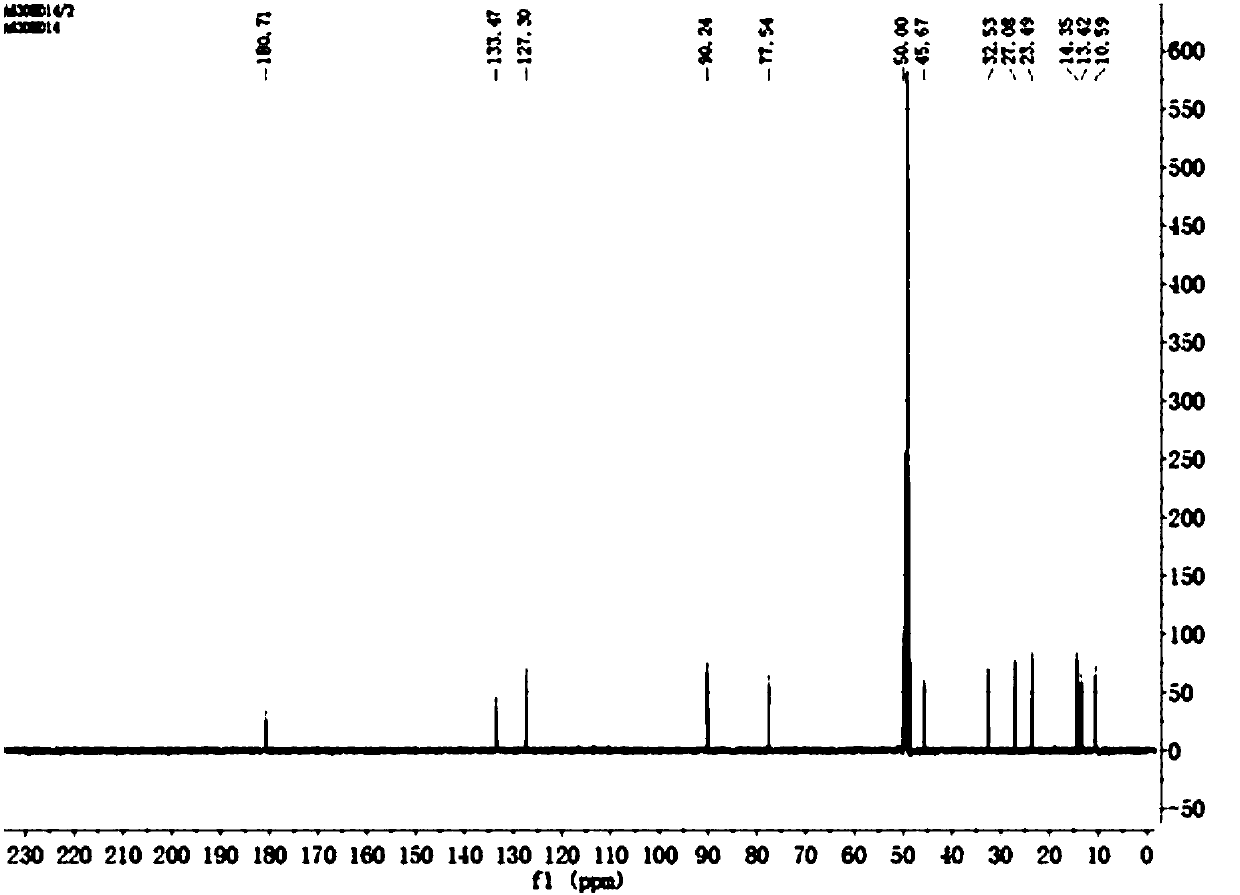
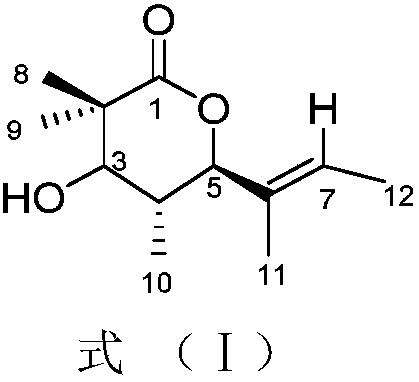
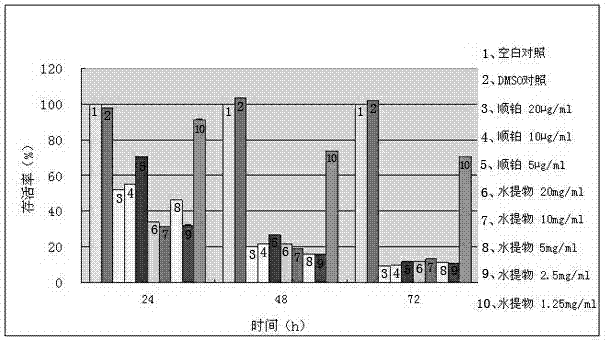
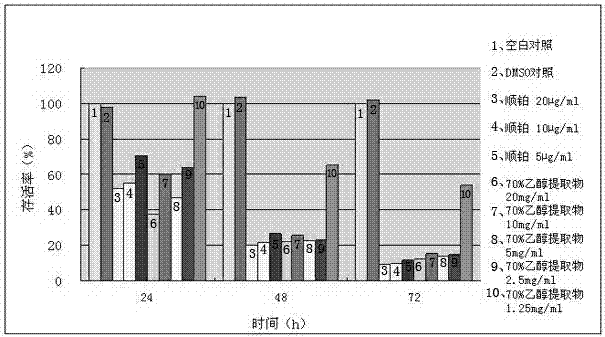
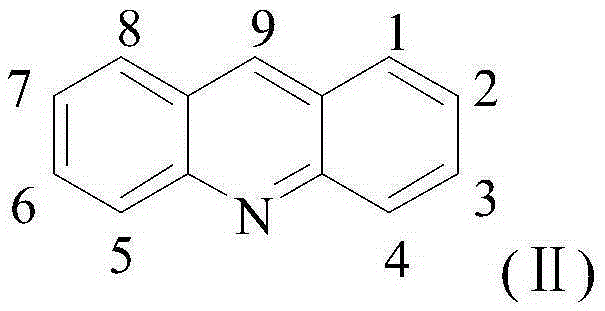
The invention discloses a platinum (II) metal complex for specifically inhibiting proliferation of a lung carcinoma cell, and a synthesis method and an application thereof. The structural formula of the platinum (II) metal complex for specifically inhibiting the proliferation of the lung carcinoma cell is as shown in formula (I). The synthesis method of the platinum (II) metal complex for specifically inhibiting the proliferation of the lung carcinoma cell includes: fetching a compound as shown in following formula (II) and dichlorodi(dimethyl sulfoxide)platinate(II), dissolving the compound and the dichlorodi(dimethyl sulfoxide)platinate(II) in polar solvent, reacting under a heating or non-heating condition, and then obtaining reaction liquid containing the target product. The metal complex can specifically inhibit the proliferation of the lung carcinoma cell NCI-H460, and IC50 (half maximal inhibitory concentration) value is up to 5.01+ / -0.54 microns, and the metal complex embodies good potential pharmaceutical value, and is hopefully used in preparation of specific lung cancer resisting pharmaceuticals. The formula (I) and the formula (II) show structures as follows.
Owner:GUANGXI NORMAL UNIV
Application of flavonoid
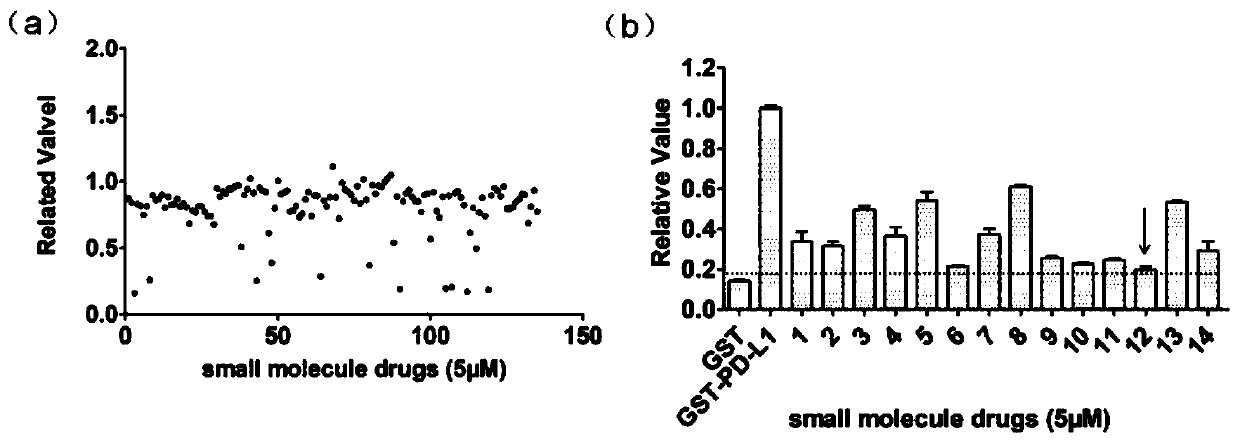
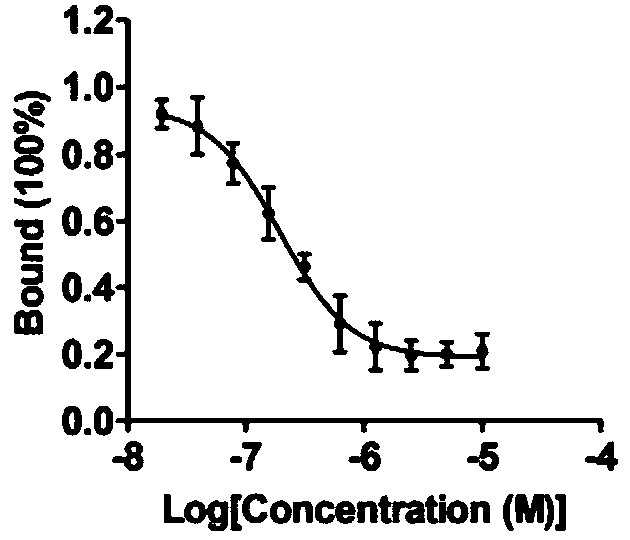
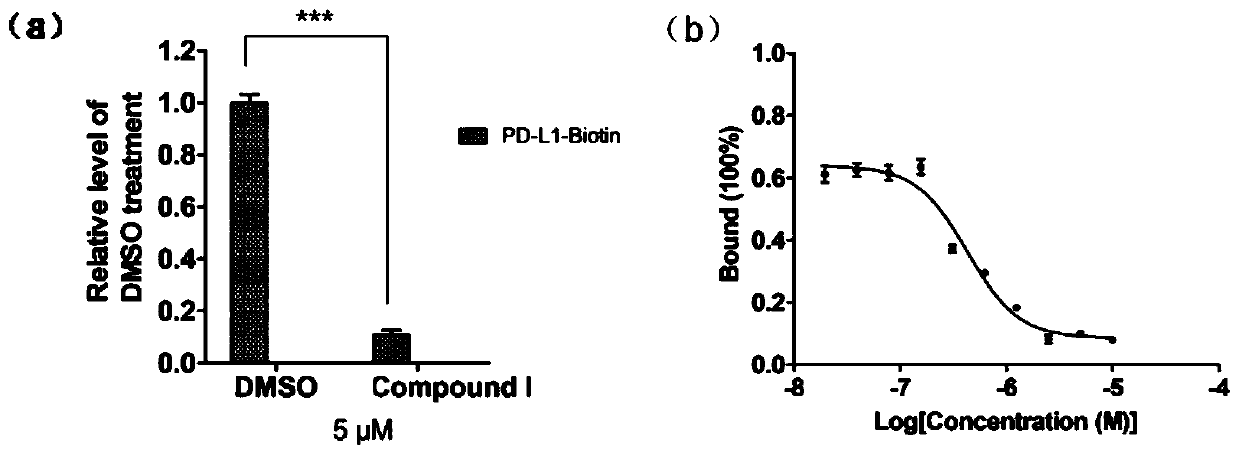
InactiveCN110200959AAvoid interactionHigh affinityOrganic active ingredientsAntineoplastic agentsMda mb 231PD-L1
The invention relates to an application of a flavonoid compound, the flavonoid compound is 3, 3', 4', 5, 7-pentahydroxyflavone dihydrate, the ELISA and protein fluorescence quenching experiments provethat the compound can effectively inhibit interaction of PD-1 / PD-L1 proteins, and has a strong affinity for PD-L1 protein. Cell clone number formation and ELISA detection of IL-2 secretion assay prove that the compound can effectively enhance the tumor killing activity of T lymphocyte Jurkat cells against MDA-MB-231 and NCI-H460 tumor cells as well as the expression level of IL-2, and then reverse T cellular immunosuppression. The research results of the application provide a drug for tumor immunotherapy targeting the PD-1 / PD-L1 immunological test site.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Marine bacillus polypeptide and preparation and application thereof
InactiveCN104610432ASolve key problemsStrong cytotoxicityPeptide/protein ingredientsMicroorganism based processesMetaboliteCytotoxicity
The invention discloses a marine bacillus polypeptide and a preparation and an application thereof and belongs to the technical field of marine microorganism medicines. The marine bacillus polypeptide is from metabolites of antarctic marine bacillus N11-8 subjected to fermentation cultivation; 15 amino acid sequences at N-ends of the active polypeptide are ASTGSQKVTVYAVAD; the active polypeptide has relatively high cytotoxicity to a plurality of tumor cells such as human hepatoma carcinoma cell BEL-7402, human ovarian carcinoma cells NIH:OVCAR-3, human renal clear cell carcinoma cells 786-0 and human large cell lung cancer cells NCI-H460, and has relatively good research and application value. The polypeptide can be applied to medicines for preventing and / or treating the cancers.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Compound Chinese actinidia root Chinese medicinal composition and preparation method and application thereof
InactiveCN102204974AGood effectHas a tumor suppressive effectAntineoplastic agentsPlant ingredientsActinidiaHep G2
The invention discloses a compound Chinese actinidia root Chinese medicinal composition and a preparation method and application thereof, belonging to the technical field of traditional Chinese medicines. The Chinese medicinal composition is characterized by comprising the following traditional Chinese medicines in parts by weight: 40-80 parts of Chinese actinidia root, 20-40 parts of salvia chinensis, 20-40 parts of herba scutellariae barbatae, 20-40 parts of herba oldenlandiae and 20-40 parts of giant knotweed. The compound Chinese actinidia root Chinese medicinal composition has remarkable proliferation inhibition effects on various cancer cells such as a hepatoma cell line Hep-G2, a lung cancer cell line NCI-H460, a gastric cancer cell line MGC-803, a breast cancer cell line MCF-7, a colon cancer cell line HCT-116 and the like, and can be used for reducing physical and psychological pains of a cancer patient in the chemo-treatment process and solving the problems of difficult and expensive administration.
Owner:ZHEJIANG SIXIAN PHARMA
Ruthenium and rhodium metal complexes taking lysicamine as ligands as well as synthetic method and application of ruthenium and rhodium metal complex
InactiveCN104744520AReduced activitySignificant in vitro antitumor activityOrganic active ingredientsRuthenium organic compoundsCytotoxicityStructural formula
The invention discloses two novel ruthenium and rhodium metal complexes taking lysicamine as ligands as well as a synthetic method and application of the two novel ruthenium and rhodium metal complexes. The synthetic method of the ruthenium and rhodium metal complexes comprises the following steps: dissolving dichloro.tetra(dimethyl sulfoxide) ruthenium (II) or rhodium trichloride and lysicamine into a polar solvent, and performing complexation reaction to prepare the ruthenium and rhodium metal complexes, wherein the ruthenium and rhodium metal complexes can be synthesized by virtue of solution methods or solvothermal methods. After the applicants investigate the proliferation inhibition activity of the ruthenium and rhodium metal complexes on human tumor cell lines such as NCI-H460, HepG-2, DLD-1 and MGC80-3 and the toxicity of the ruthenium and rhodium metal complexes to human normal liver cells HL-7702, results show that the ruthenium and rhodium metal complexes have significant in-vitro anti-tumor activity, and the activity of a rhodium metal complex is equal to that of cis-platinum; moreover, the ruthenium and rhodium metal complexes have relatively low cytotoxicity to normal cells, have good potential medicinal values, and are expected to be applied to the preparation of various anti-tumor medicines; and structural formulas of the complexes are respectively shown in the following formula (I) and formula (II) in the specification.
Owner:GUANGXI NORMAL UNIV
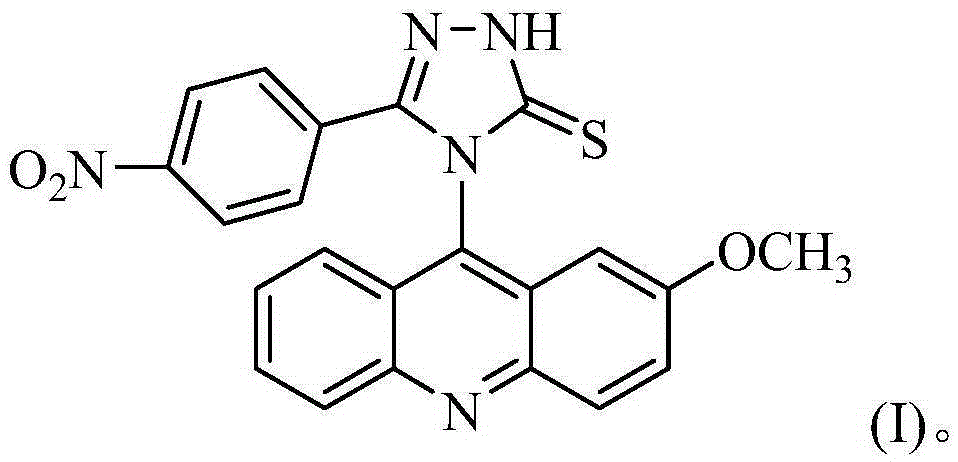
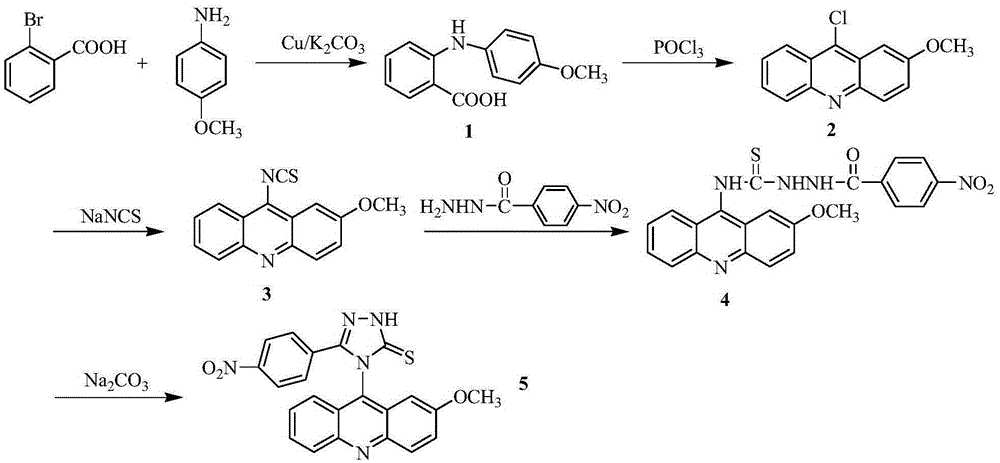
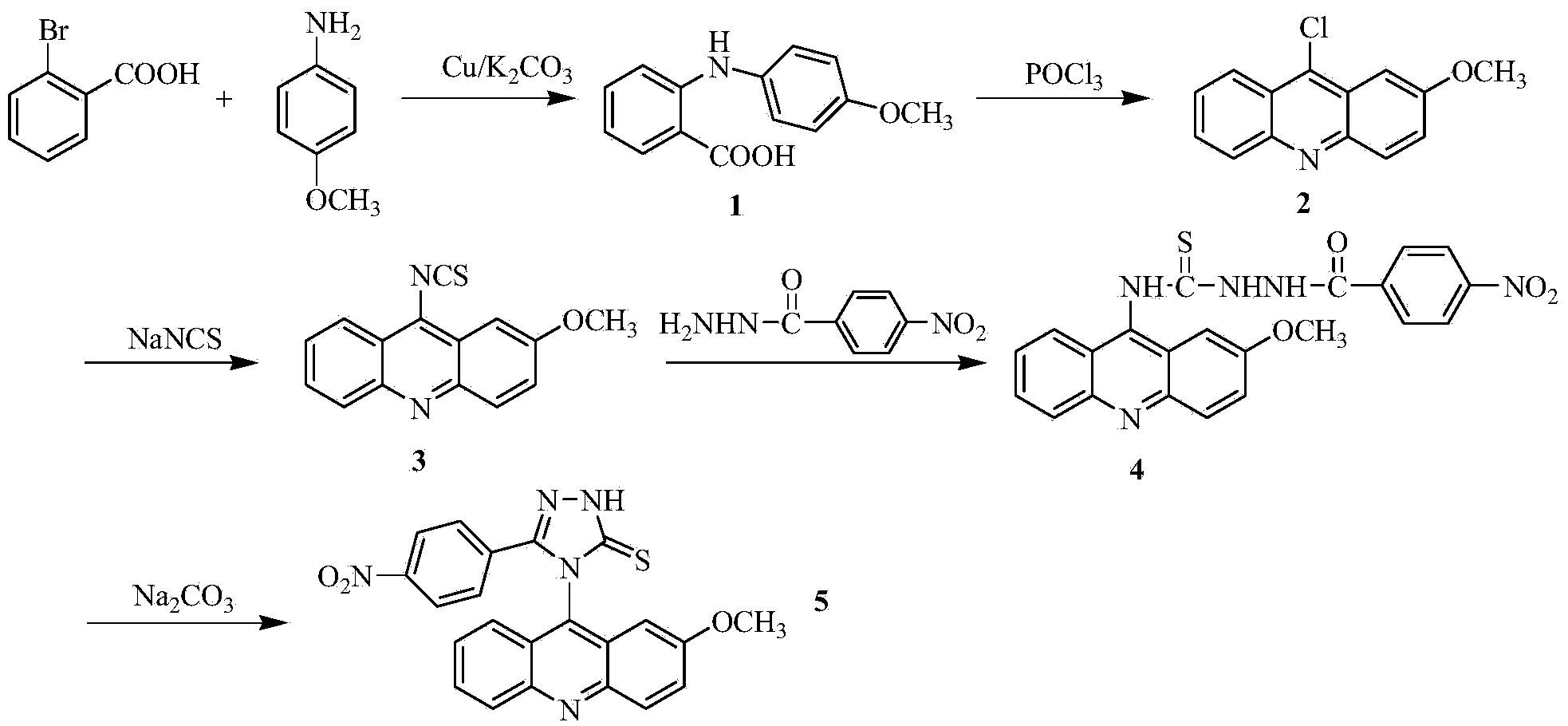
Acridine-1,2,4-triazole-5-thioketone compound and preparation method and applications of acridine-1,2,4-triazole-5-thioketone compound
ActiveCN104277028AIncrease the conjugate areaEasy to embedOrganic active ingredientsOrganic chemistrySodium sulfocyanateThioketone
The invention discloses an acridine-1,2,4-triazole-5-thioketone compound and a preparation method and applications of the acridine-1,2,4-triazole-5-thioketone compound. The preparation method of the compound comprises the following steps: 1) by taking an o-bromobenzoic acid and p-methoxyaniline as raw materials, taking potassium carbonate and copper powder as catalysts, and taking isopentyl alcohol or n-amyl alcohol as a solvent, reacting so as to obtain a compound 1; 2) carrying out cyclization on the compound 1 by using phosphorus oxychloride so as to obtain a compound 2; 3) after the compound 2 is dissolved by using an organic solvent, in the presence of tetrabutylammonium bromide, reacting the dissolved compound 2 with sodium sulfocyanate so as to obtain a compound 3; 4) after the compound 3 is dissolved by using an organic solvent, reacting the dissolved compound 3 with m-nitrobenzoylhydrazine so as to obtain a compound 4; and 5) reacting the compound 4 with sodium carbonate, carrying out suction filtration on a reactant, collecting filter liquor, adjusting the pH value of the filter liquor to be less than 4, separating out precipitates, and carrying out suction filtration on the precipitates. In-vitro antitumor test results show that the compound has a significant in-vitro antitumor activity to tested MGC80-3, NCI-H460 and T24.
Owner:广西新桂环保科技集团有限公司
Shikonin carbohydrate derivatives and synthetic method and use thereof
InactiveCN101392010AStrong inhibitory activityOrganic active ingredientsSugar derivativesCarbohydrate derivativeChemical synthesis
The invention belongs to the field of chemical pharmaceutical technology, particularly relates to drug design of the traditional Chinese medicine extract shikonin serving as a lead compound and related research of anti-tumor activity. Carbohydrates are led into the framework molecule of the shikonin by means of chemical synthesis so as to obtain carbohydrate derivative with novel structure, and the research of in-vitro anti-tumor activity shows that the inhibition activity of the glycosyl shikonin on tumor cell A549 is obviously better than that of NCI-H460. From the activity result, the glycosyl shikonin can be prepared into anti-tumor medicament with high effect and low toxicity.
Owner:NANJING UNIV
(2-pyridinecarbaldehyde)-2,6 pyridine bisacylhydrazone copper compound, preparation method and application thereof
InactiveCN105777783BHigh anticancer activityIncrease fat solubilityOrganic active ingredientsGroup 1/11 organic compounds without C-metal linkagesSolubilityPlatinum
The invention discloses a (2-pyridylaldehyde)-2,6 pyridine diacylhydrazone copper compound and a preparation method.The copper compound has high anti-cancer activity and can be used as the raw material for preparing medicine for treating human breast cancer cells (MDA) and Human large cell lung cancer (NCI-H460).Compared with platinum anti-cancer medicine commonly used at present, the copper compound has the advantages of being high in anti-cancer activity, good in lipid solubility, low in cost, simple in preparation method and the like, and provides a new means for anti-cancer medicine development.
Owner:LIAOCHENG UNIV
Monocarbonyl curcumin analogs, and preparation method and application thereof
InactiveCN105693492AImprove stabilitySimple structureOrganic compound preparationCarbonyl compound preparationBenzaldehydeApoptosis
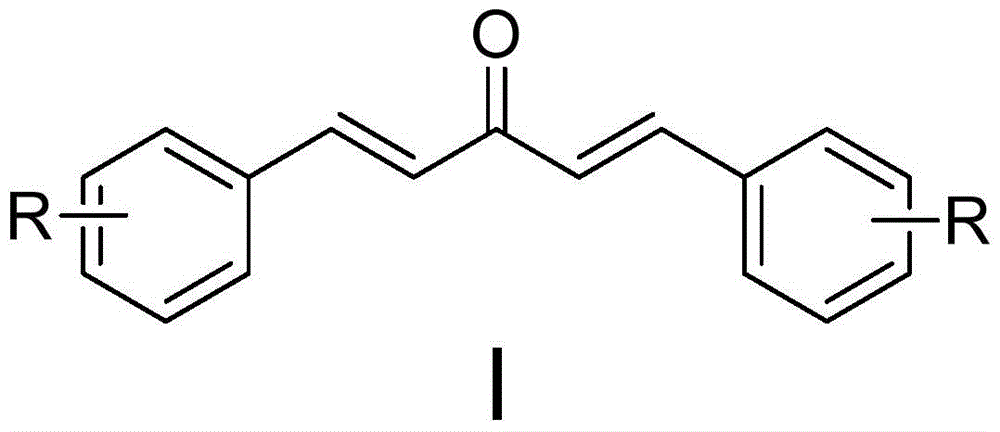
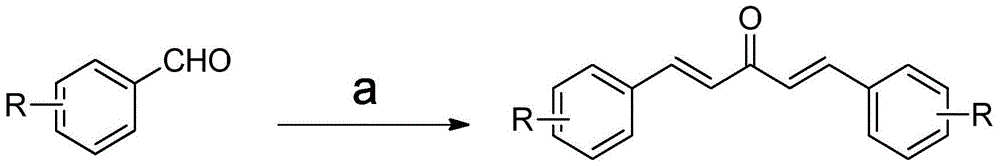
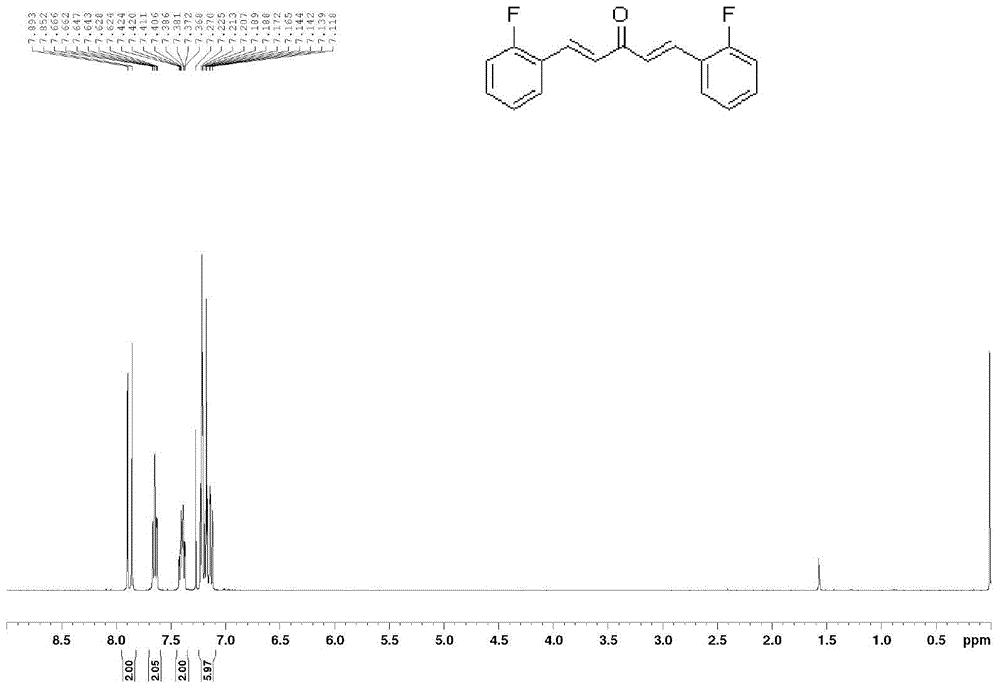
The invention discloses a monocarbonyl curcumin analogue, a preparation method and application thereof. The analogue has the structural features of general formula (I), wherein R is fluorine, the substitution positions of R are 2, 3, and 4-positions, and the two benzene rings pass through 1,4-pentadien-3-one connect. The invention uses o-, m-, p-fluorine substituted benzaldehyde as raw materials to generate monocarbonyl curcumin analogs through condensation with acetone. The stability of 2,2'-fluoromonocarbonyl curcumin in medium containing 10% serum is better than that of natural product curcumin. The results of pharmacological experiments showed that the anti-proliferation activity of fluorine-substituted monocarbonyl curcumin on human lung cancer NCI-H460 cells was significantly superior to that of natural product curcumin, and 2,2'-fluoro monocarbonyl curcumin could lead to cell proliferation by promoting the production of reactive oxygen species. Redox imbalance, lipid peroxidation, breakdown of mitochondrial membrane potential, and apoptosis in the body can be used as curcuminoid leads for the treatment of lung cancer.
Owner:LIAOCHENG UNIV
Preparation method for isochromophilone VIII and application of same in preparation of antineoplastic drugs
The invention discloses a preparation method for isochromophilone VIII and application of the same in preparation of antineoplastic drugs. According to the invention, isochromophilone VIII with antineoplastic activity is isolated from a liquid fermentation culture of the marine fungus Penicillium sp. FS60. According to results of experiments, it is found that the IC50 values of isochromophilone VIII in inhibiting SF-268 cells, MCF-7 cells and NCI-H460 cells are 7.17, 6.36 and 22.27 mu g / mL, respectively; and isochromophilone VIII has significant antineoplastic activity. The invention providesa candidate drug for research and development of novel antineoplastic drugs and scientific bases for development and utilization of marine microbial resources.
Owner:GUANGDONG INST OF MICROBIOLOGY GUANGDONG DETECTION CENT OF MICROBIOLOGY
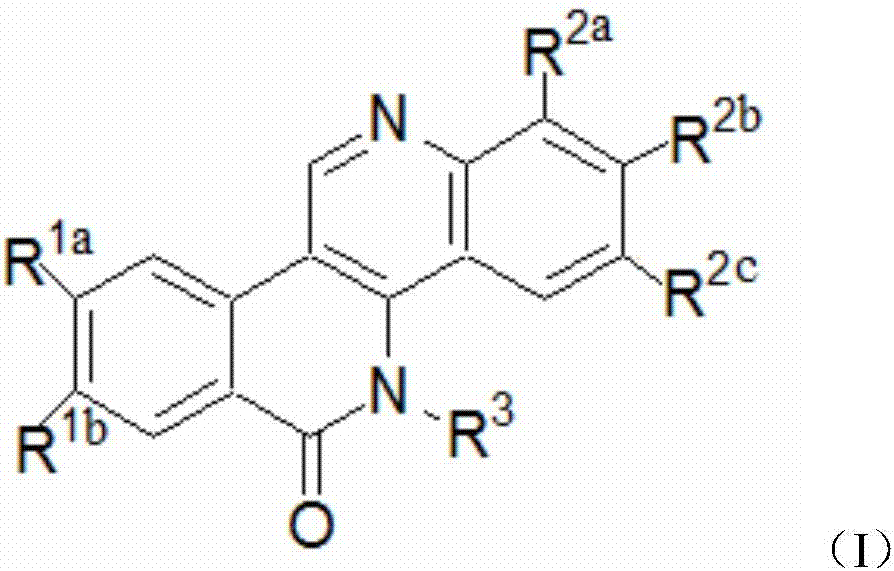
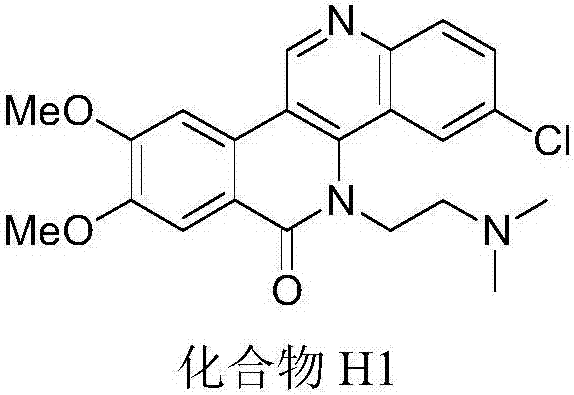
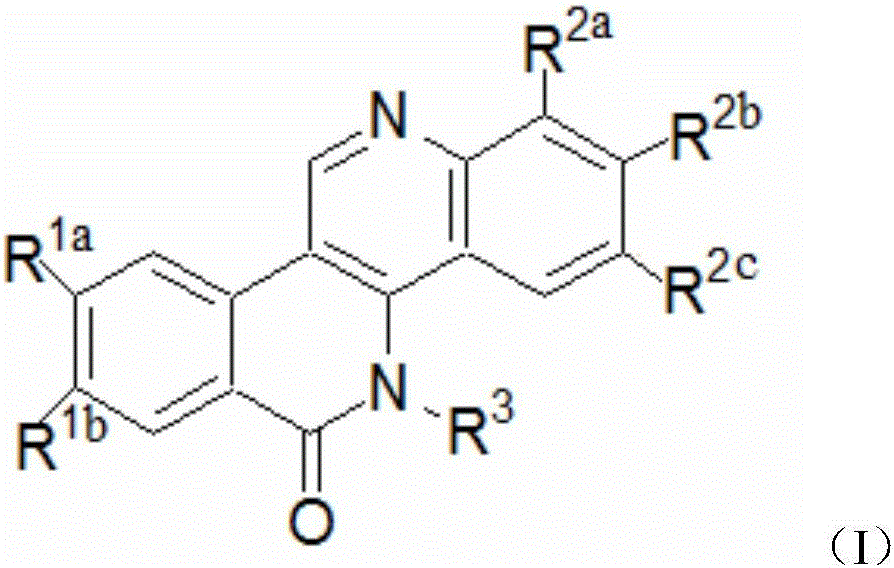
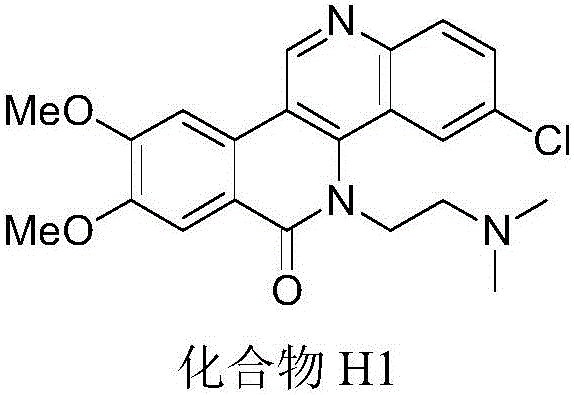
Dibenzonaphthyridinone compounds, preparation method, and applications thereof
ActiveCN106188049AStrong inhibitory activityOrganic active ingredientsOrganic chemistryCancer cellNci h460
The invention discloses dibenzonaphthyridinone compounds represented by the formula (I), which is shown in the description. In the formula (I), R1a is independently chosen from -H and -CH3O, R1b is -CH3O; when R2a is -Cl, R2b and R2c are both -H; when R2b is -Cl, R2a and R2b are both -H; when R2c is independently chosen from -F, -Cl, -Br, -CH3, -CH3O, and -CF3, R2a and R2b are both -H; and R3 is (CH2)2N(CH3)2 or (CH2)3N(CH3)2. The invention further discloses a preparation method of dibenzonaphthyridinone compounds and an application of dibenzonaphthyridinone compounds in the preparation of anti-tumor drugs. The test results show that dibenzonaphthyridinone compounds have a good effect on inhibiting the activity of cells such as human stomach cancer cell (MGC-803), human lung cancer cell (NCI-H460), human liver cancer cell (HepG-2), human liver cancer cell (BEL-7404), and the like, and thus has a good application prospect in antitumor industry.
Owner:GUANGXI NORMAL UNIV
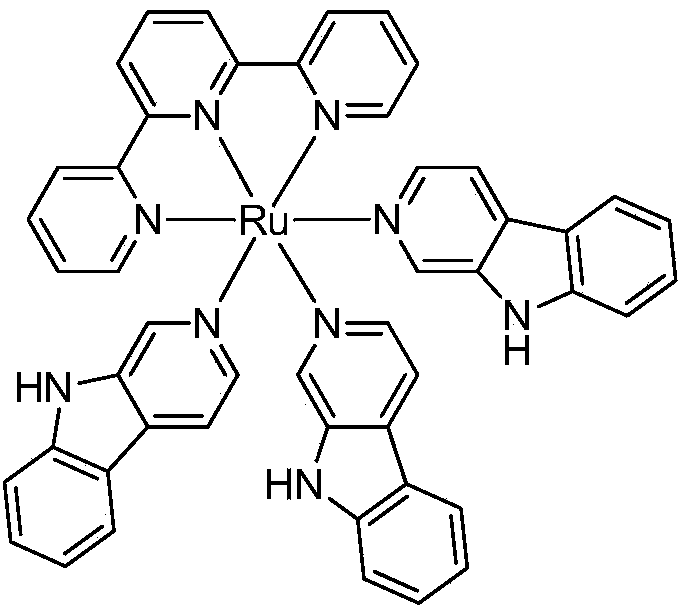
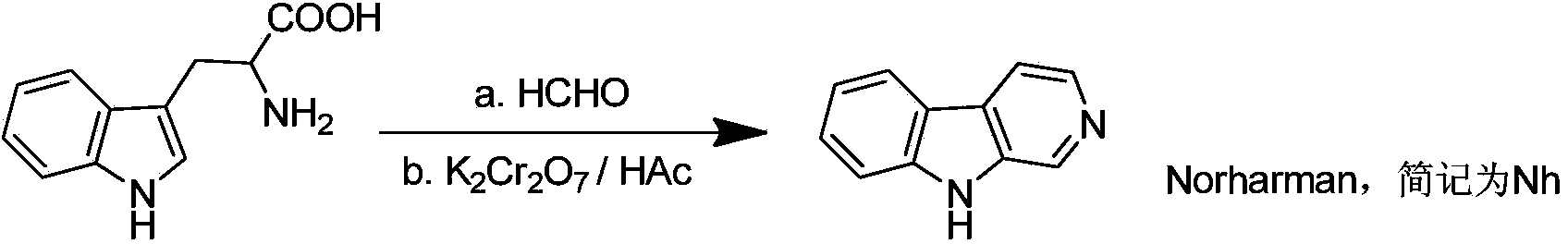
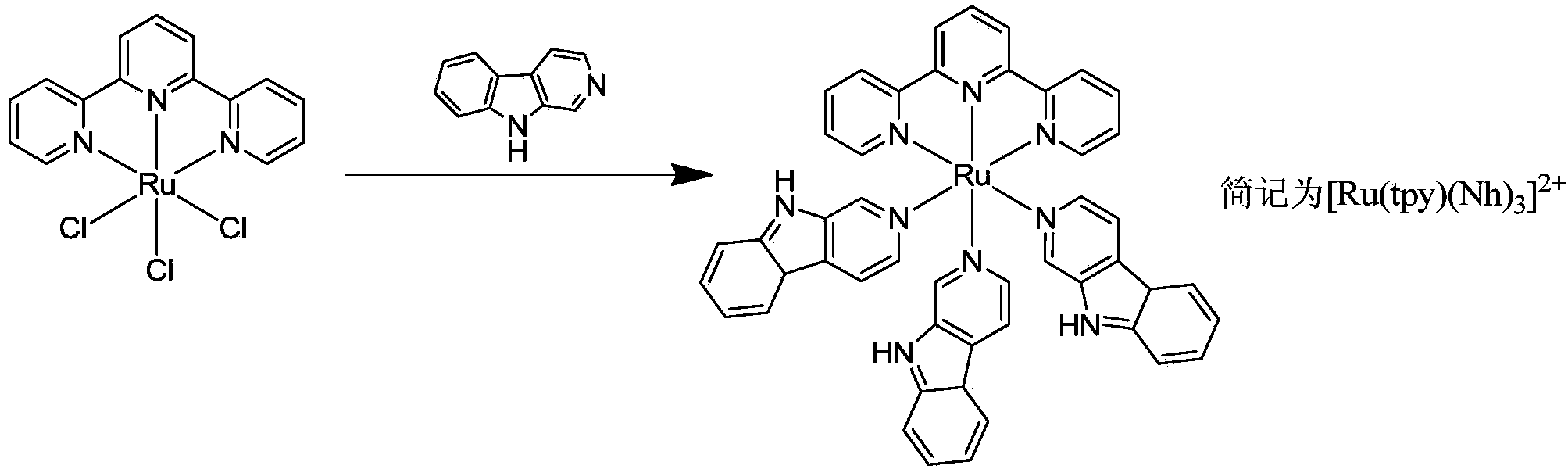
Norharman-ruthenium (II) polypyridine complex with antitumour activity
InactiveCN103483388AStable structureEnhance cell transmembrane abilityGroup 8/9/10/18 element organic compoundsAntineoplastic agentsChemistryNci h460
The invention discloses a preparation method for a Norharman-ruthenium (II) polypyridine complex and the antitumour activity thereof. The chemical formula of the Norharman-ruthenium (II) polypyridine complex disclosed by the invention is [Ru(tpy)(Norharman)3]<2+>(tpy=2,2':6',2''- terpyridyl, Norharman=9H-pyridino-[3,4-b] indol), and prepared by taking ruthenium trichloride and Norharman as raw materials, and performing the steps of heating reflux reaction, cooling and filtering, washing, drying and the like. The influence of the complex on tumour cells is researched in in-vitro antitumour activity test, and the complex is found to be strong in an inhibition effect on the growth of tumour cells HeLa, HepG2, A549, MCF-7, NCI-H460, Bel-7402 and HCT-116. The complex is indicated to be good in a tumour inhibition effect by animal test data, and expected to be a novel antitumour medicinal member, and wide in application prospect.
Owner:SUN YAT SEN UNIV
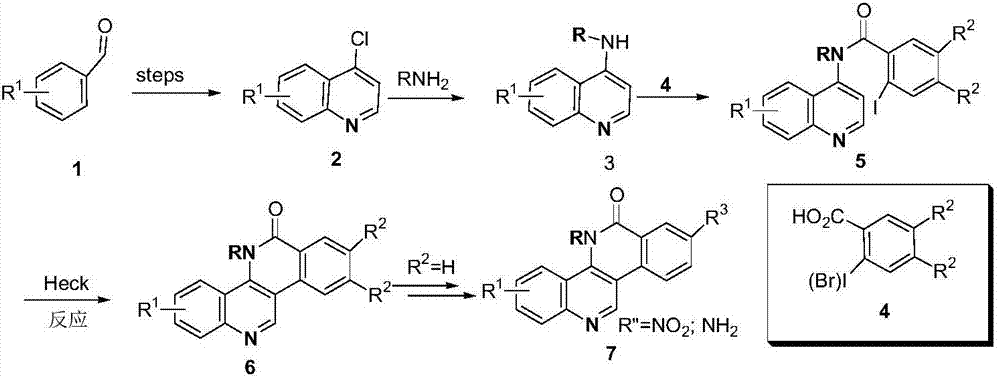
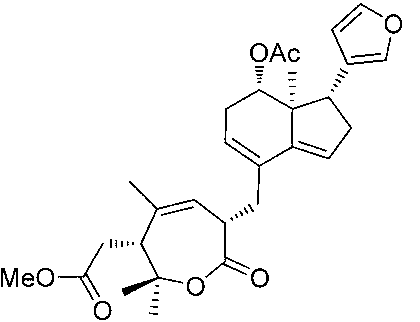
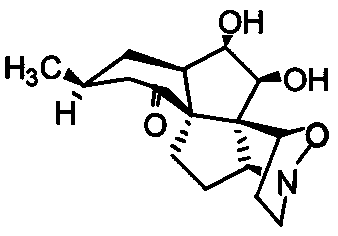
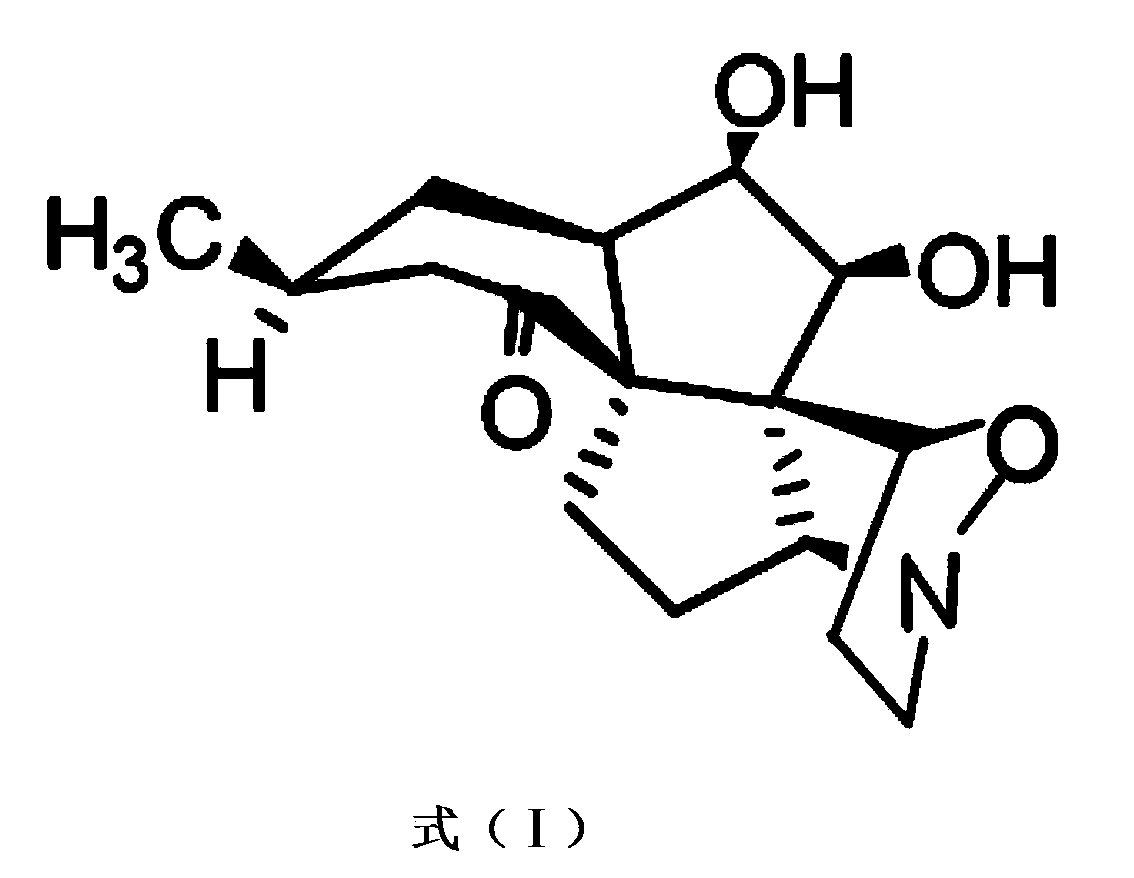
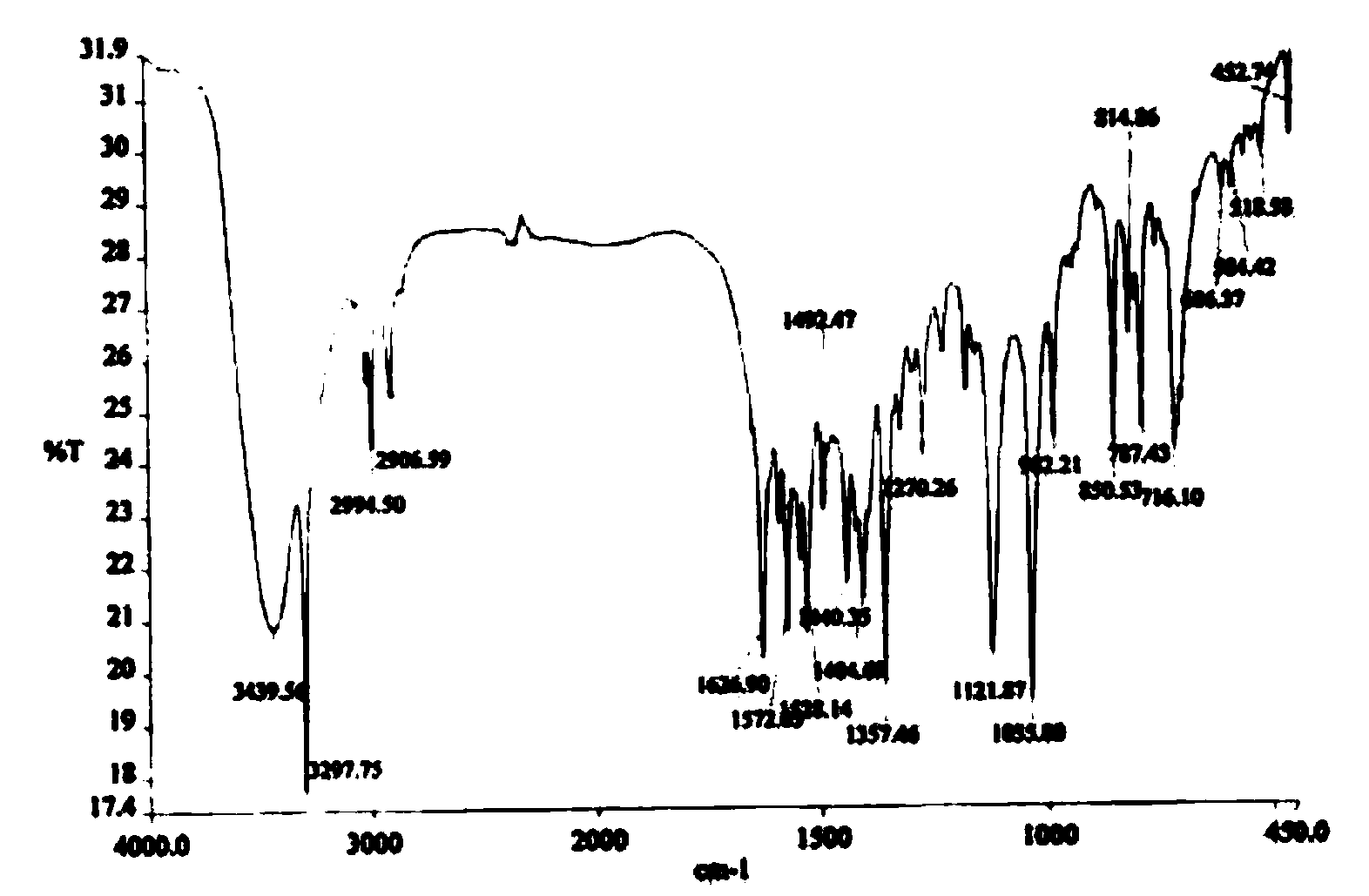
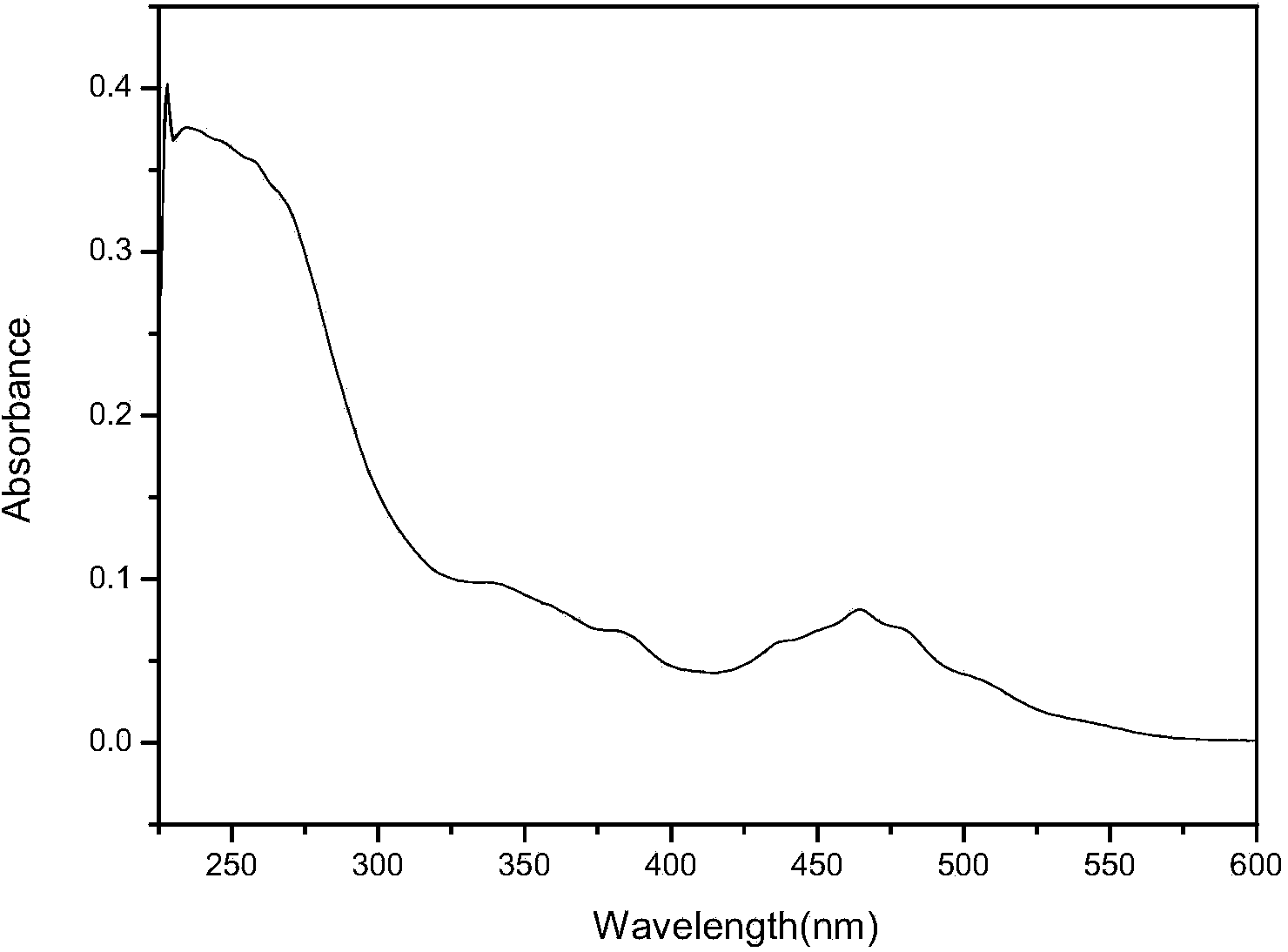
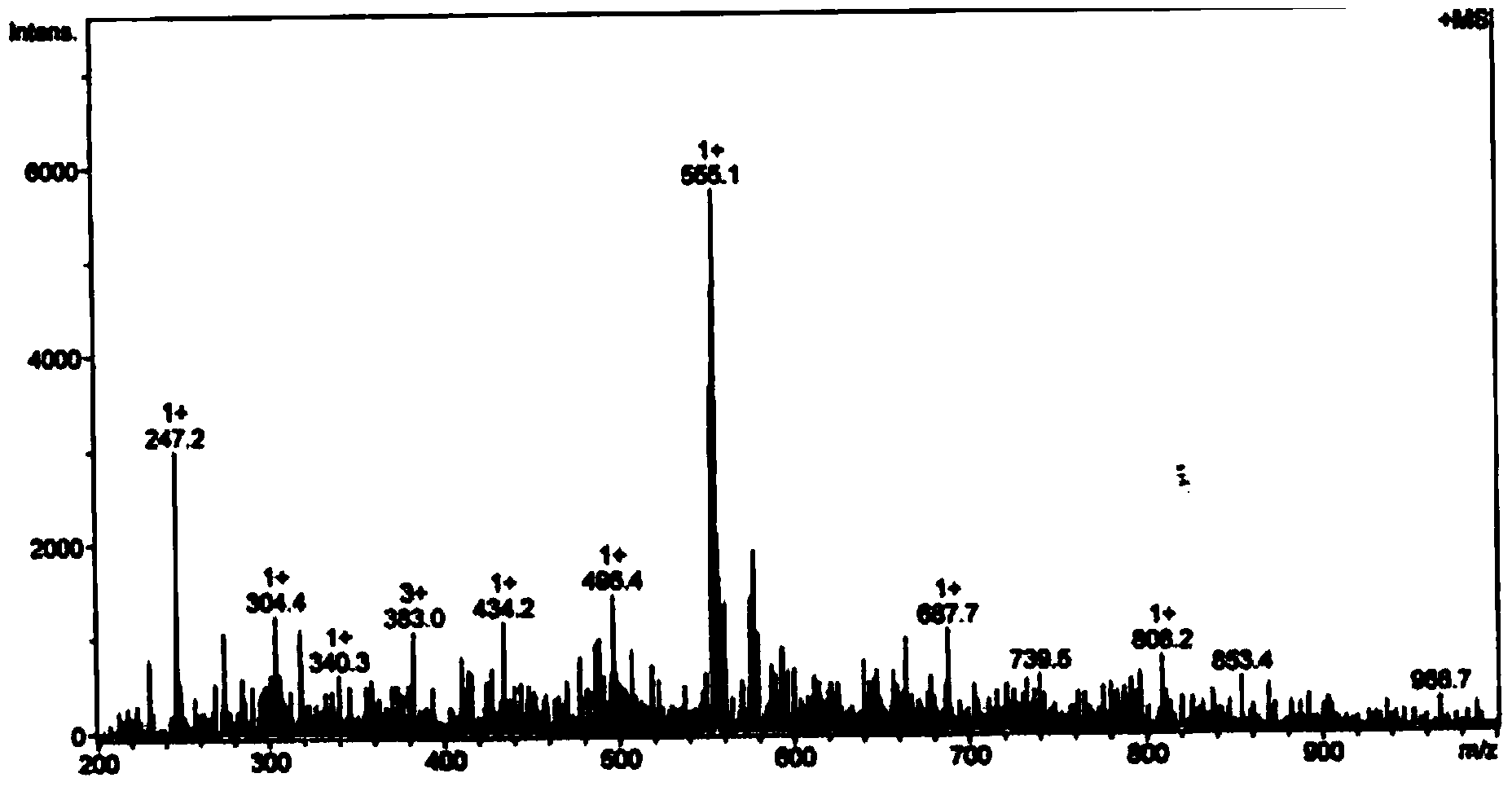
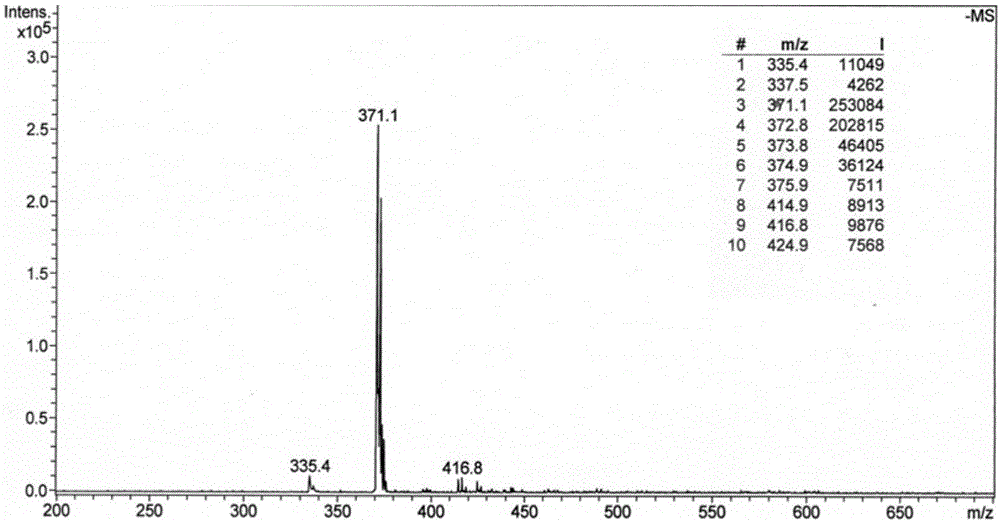
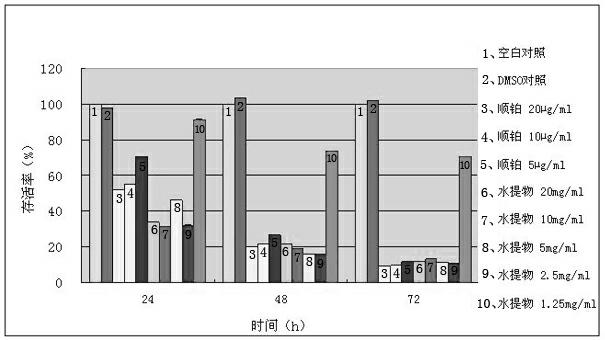
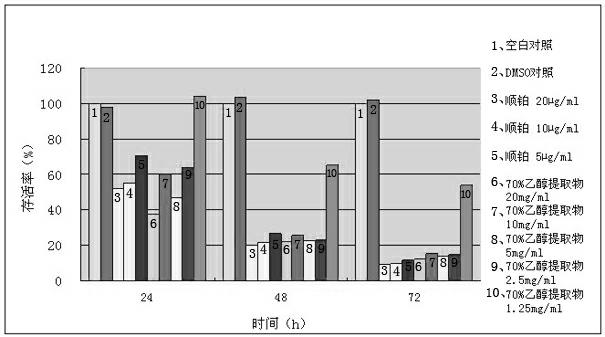
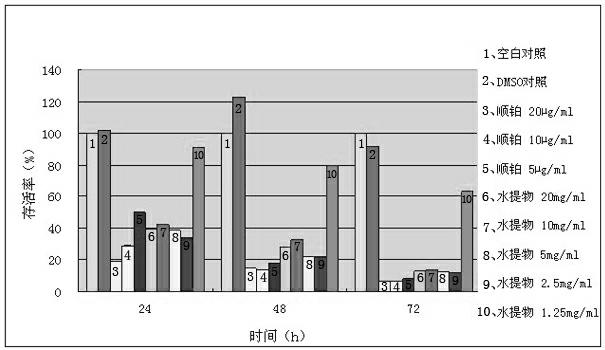
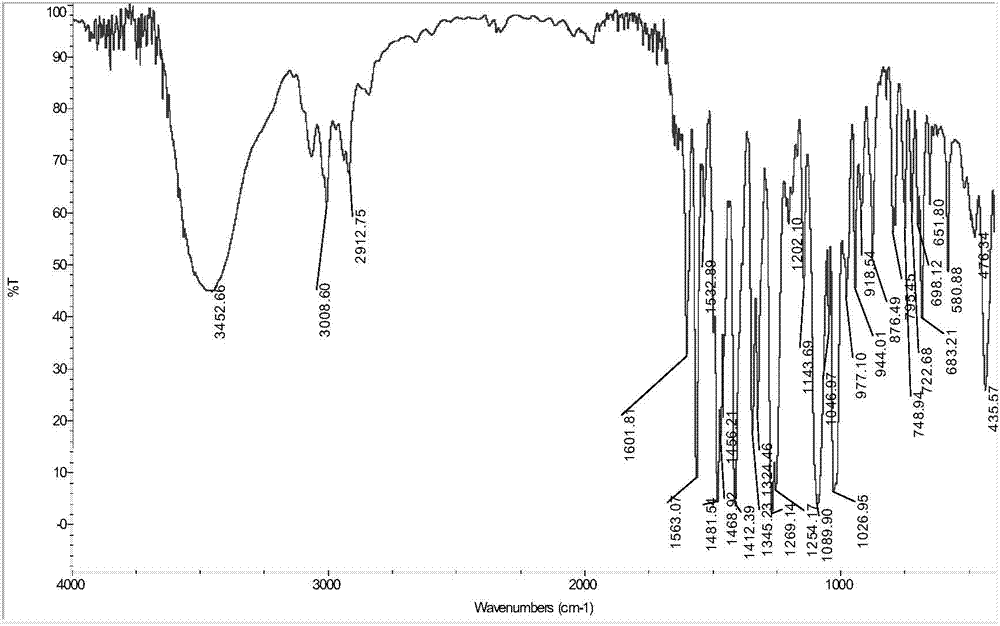
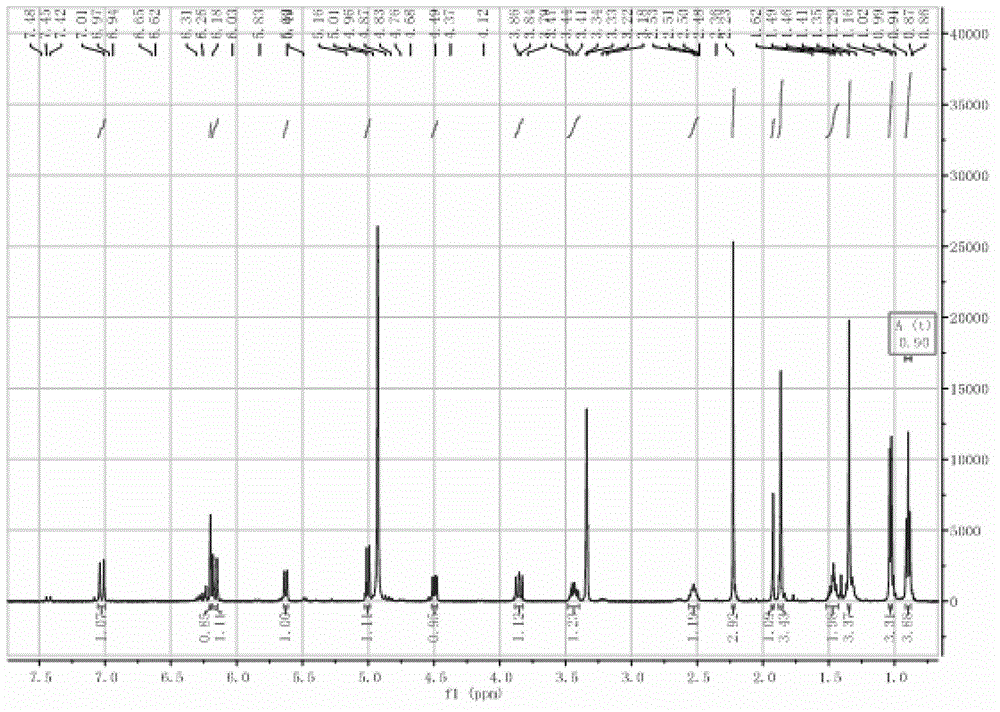
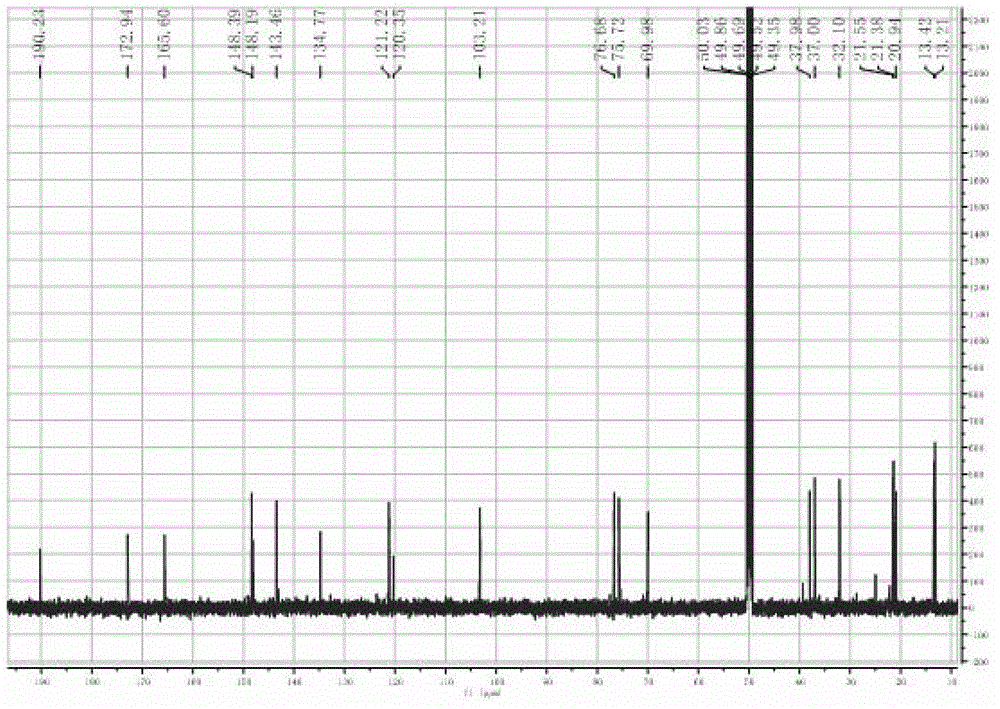
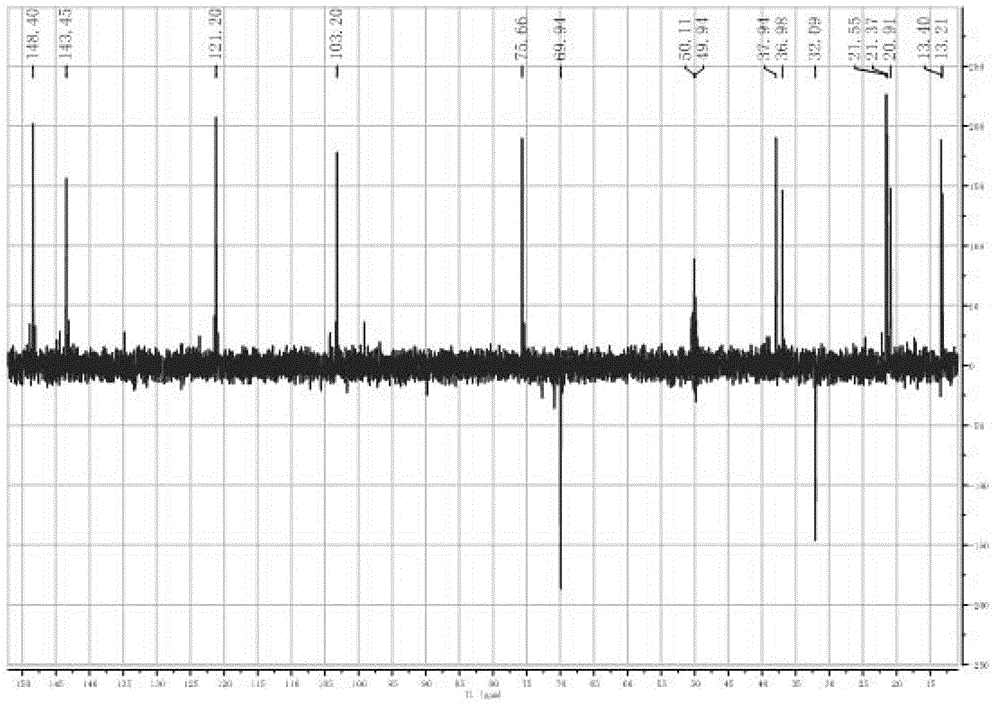
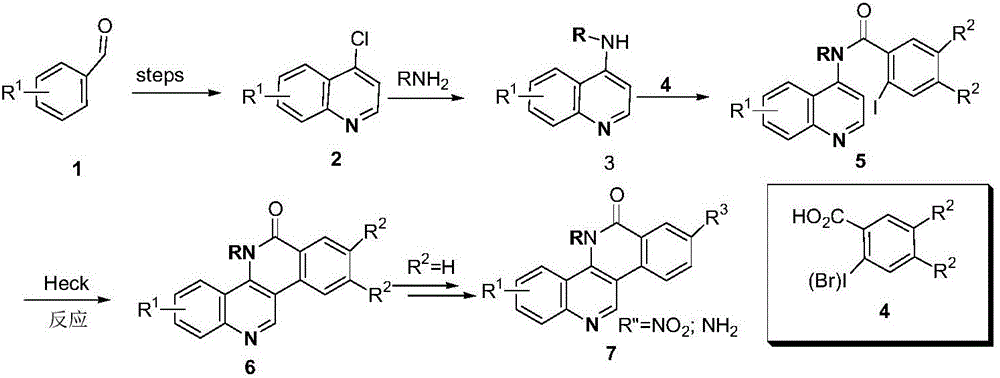
Phthalizine [1,2,b] quinazoline-8-ketone compound and preparation method and application in antitumor drugs of phthalizine [1,2,b] quinazoline-8-ketone compound
InactiveCN105461723ACan induce apoptosisEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryApoptosisHuman gastric carcinoma
The invention discloses a phthalizine [1,2,b] quinazoline-8-ketone compound, which is characterized in that the structural general formula of the compound is shown in the description, and phthalizine [1,2,b] quinazoline-8-ketone derivatives disclosed by the invention have very strong inhibiting effect on common cancer cells such as human gastric carcinoma cells (MGC-803), human lung cancer cells (NCI-H460), human hepatoma cells (HepG-2), cervical carcinoma cells (Hela), human bladder cancer cells (T-24) and the like, can induce apoptosis of the cancer cells, hence the phthalizine [1,2,b] quinazoline-8-ketone and derivatives thereof have potential application in preparation of antitumor drugs.
Owner:GUANGXI NORMAL UNIV
Application of catclaw buttercup root extract to preparation of anti-lung cancer medicines
InactiveCN102805768ASimple methodLow costAntineoplastic agentsPlant ingredientsNormal cellAdenocarcinoma lung cancer
The invention discloses application of catclaw buttercup root extracts to preparation of anti-lung cancer medicines. The anti-lung cancer medicines comprise medicines for preventing and / or treating lung cancer. The catclaw buttercup root extracts have a good anti-lung cancer effect, growth of human lung cancer cells such as human lung adenocarcinoma cells A549 and large human lung cancer cells NCI-H460 can be effectively inhibited, and the catclaw buttercup root extracts have a low toxic effect on normal cells, is high-efficient and low-toxicity, and can be used for preparing the anti-lung cancer medicines.
Owner:ZHEJIANG ACAD OF TRADITIONAL CHINESE MEDICINE
Dibenzonaphthyridinone compounds and their preparation methods and applications
ActiveCN106188049BStrong inhibitory activityOrganic active ingredientsOrganic chemistryCancer cellKetone
Owner:GUANGXI NORMAL UNIV
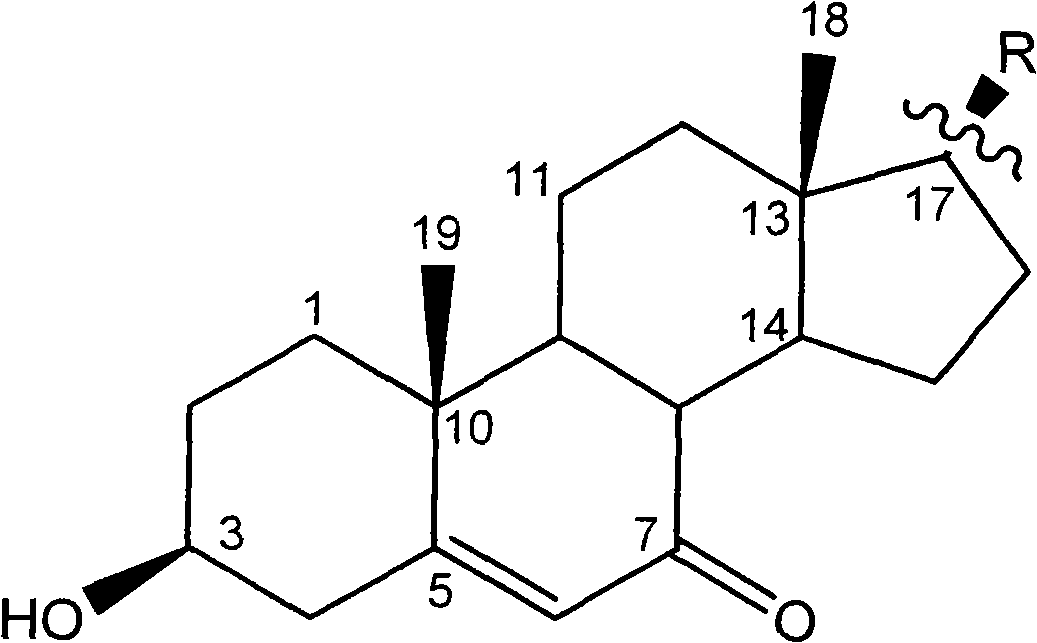
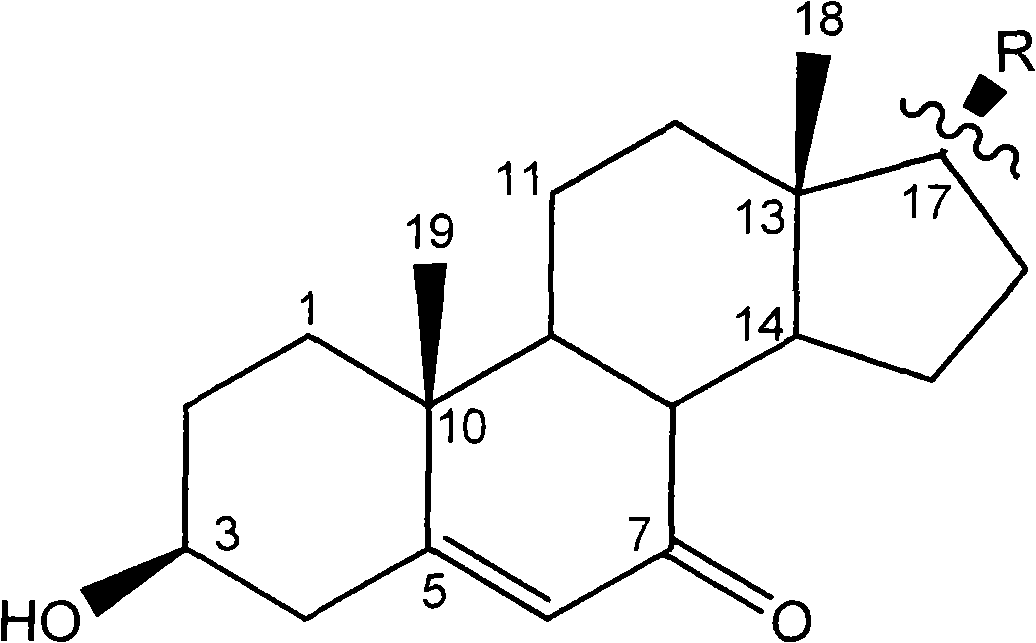
Steroidal compound in Bugula neritina L. and use thereof
InactiveCN101514222AEasy to prepareGood antitumor activityOrganic active ingredientsSteroidsChemical structureIc50 values
The invention relates to the field of medical technology and discloses a new steroidal compound which is extracted in marine animal Bugula neritina L. and the application thereof in the preparation of anti-tumor drugs. The general formula of the chemical structure thereof is as follows: the in vitro activity test proves that the steroidal compound has significant anti-tumor activity to three different tumor cells of HepG2, HT-29 and NCI-H460, and the IC50 value is close to or even better than a positive control drug which is vincristine, thereby being capable of being used for preparing the anti-tumor drugs.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Preparation method for Sanhuang tablet
InactiveCN103520294ARich in active ingredientsReduce dosageOrganic active ingredientsPill deliveryBaical Skullcap RootBerberine hydrochloride
The invention provides a preparation method for a Sanhuang tablet. The Sanhuang tablet is prepared from the bulk drugs consisting of 300 g of rhubarb root and rhizome, 5 g of berberine hydrochloride and 80 g of baical skullcap root extract through supercritical extraction, so the content of Sanhuang tablet is greatly increased, and the dose of the Sanhuang tablet is reduced. The invention further provides application of the Sanhuang tablet in preparation of drugs used for inhibiting cell proliferation of human large cell carcinoma NCI-H460.
Owner:NANJING ZHENGLIANG MEDICAL TECH
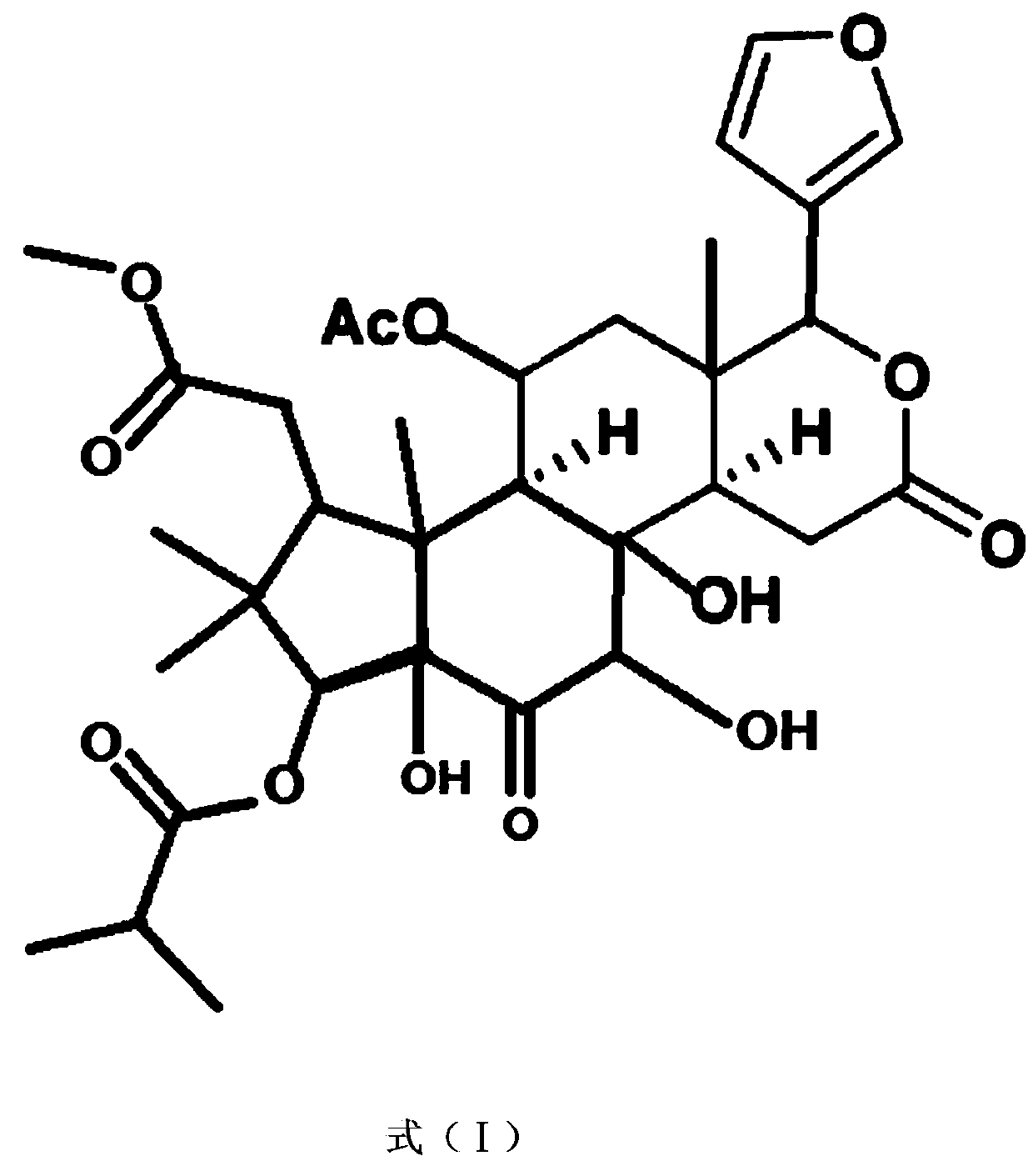
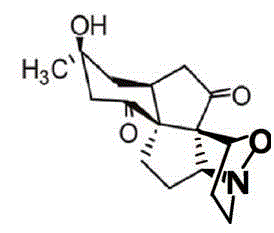
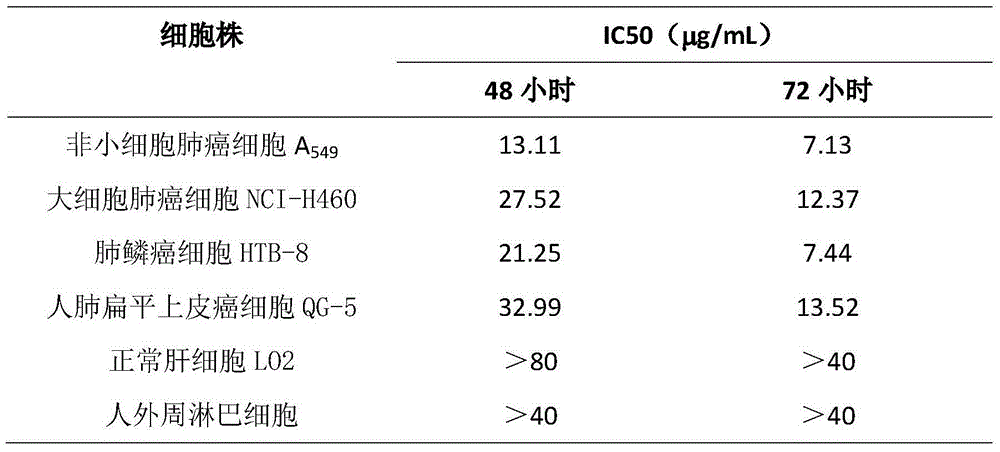
Application of Aphanamixoid A to lung cancer treatment medicine
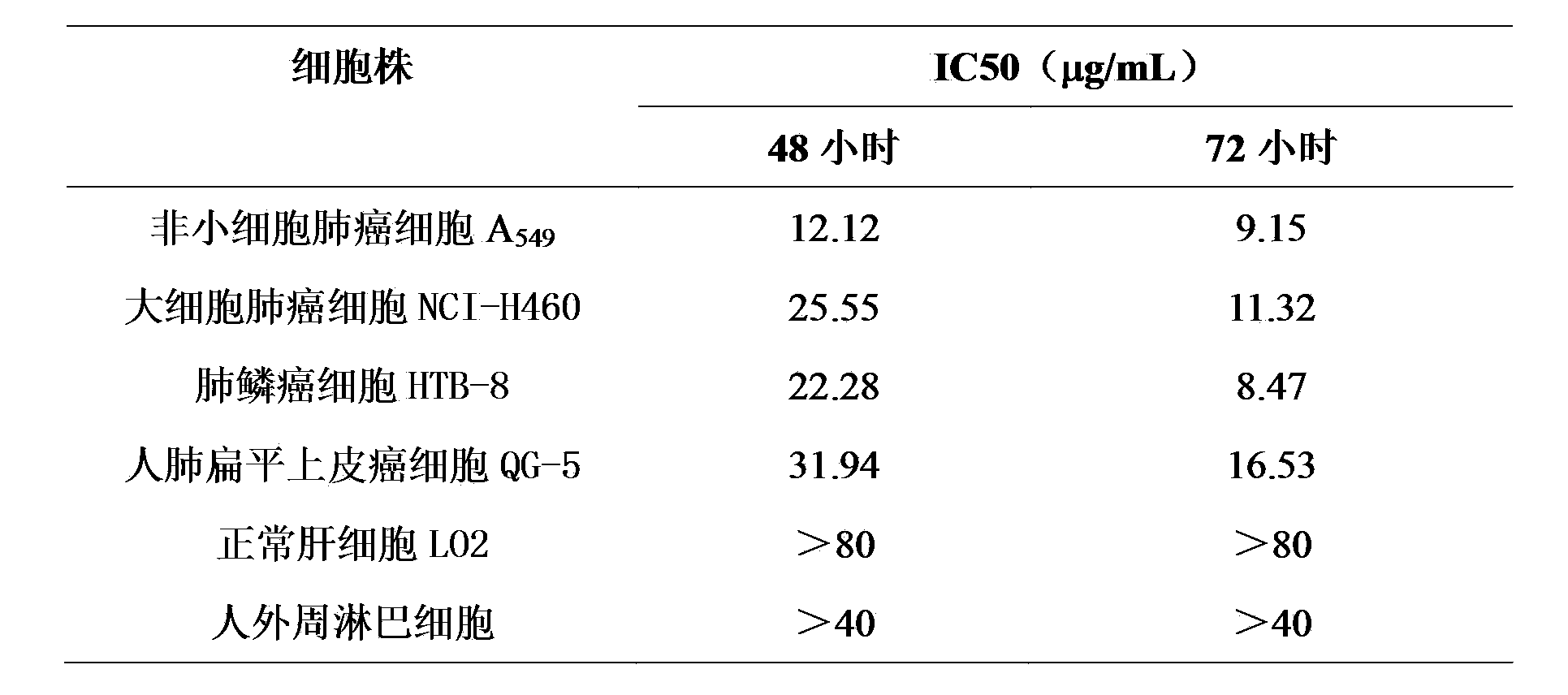
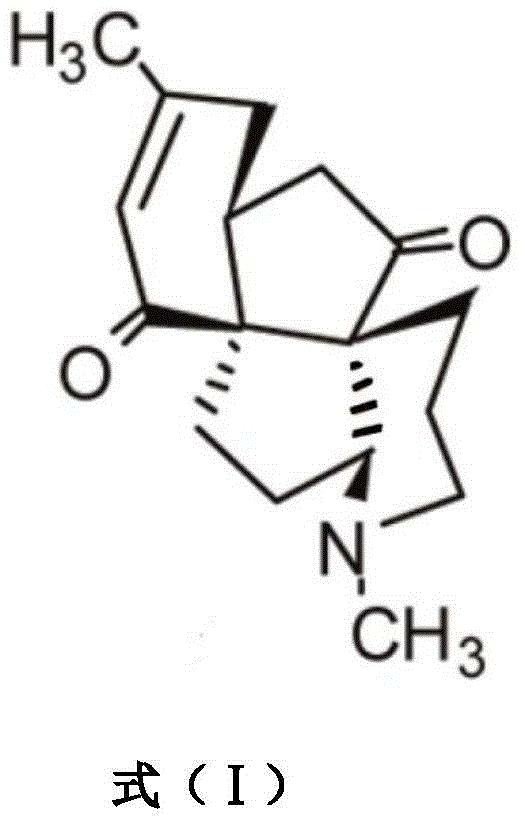
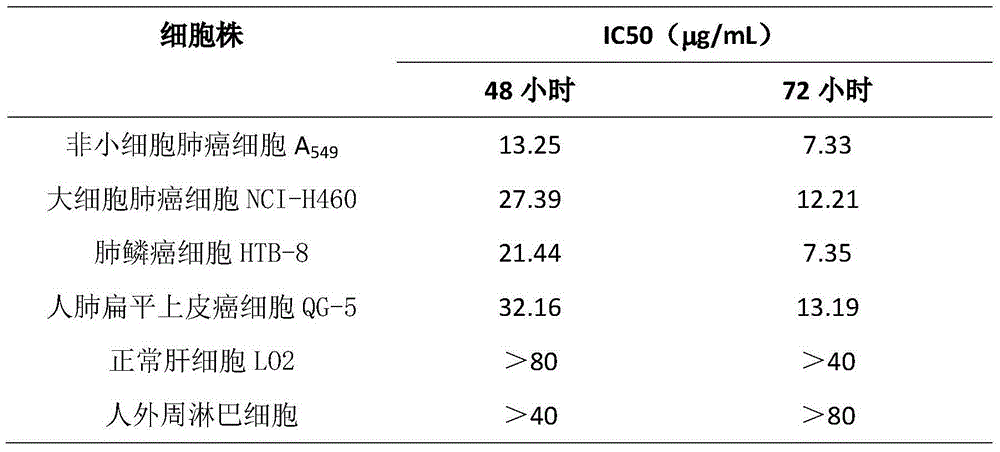
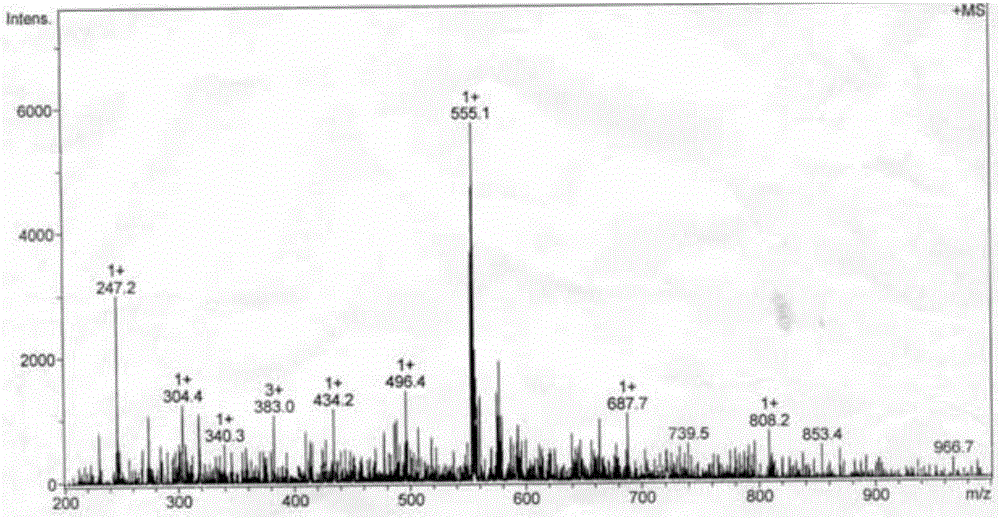
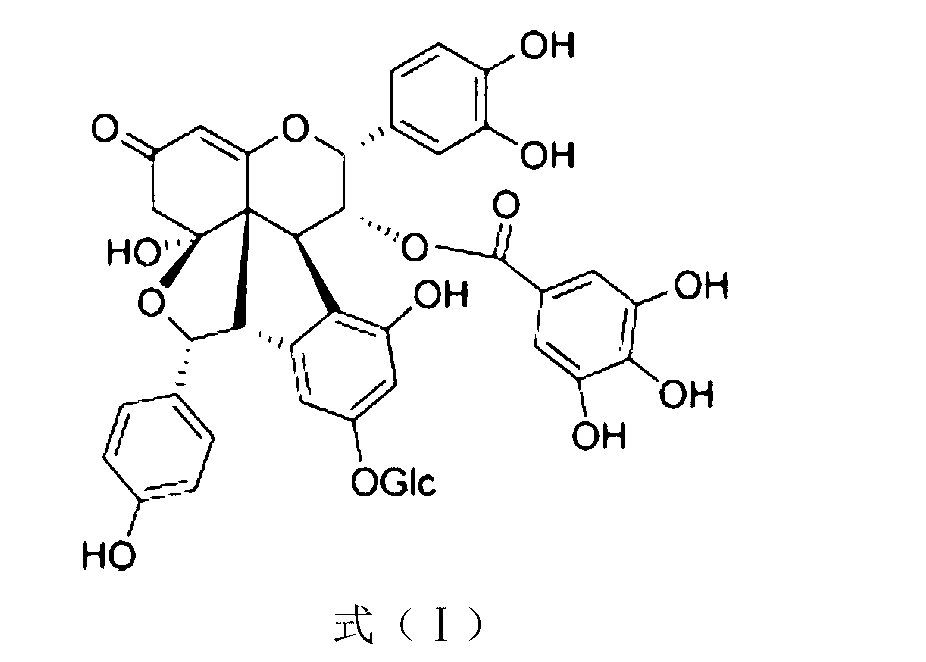
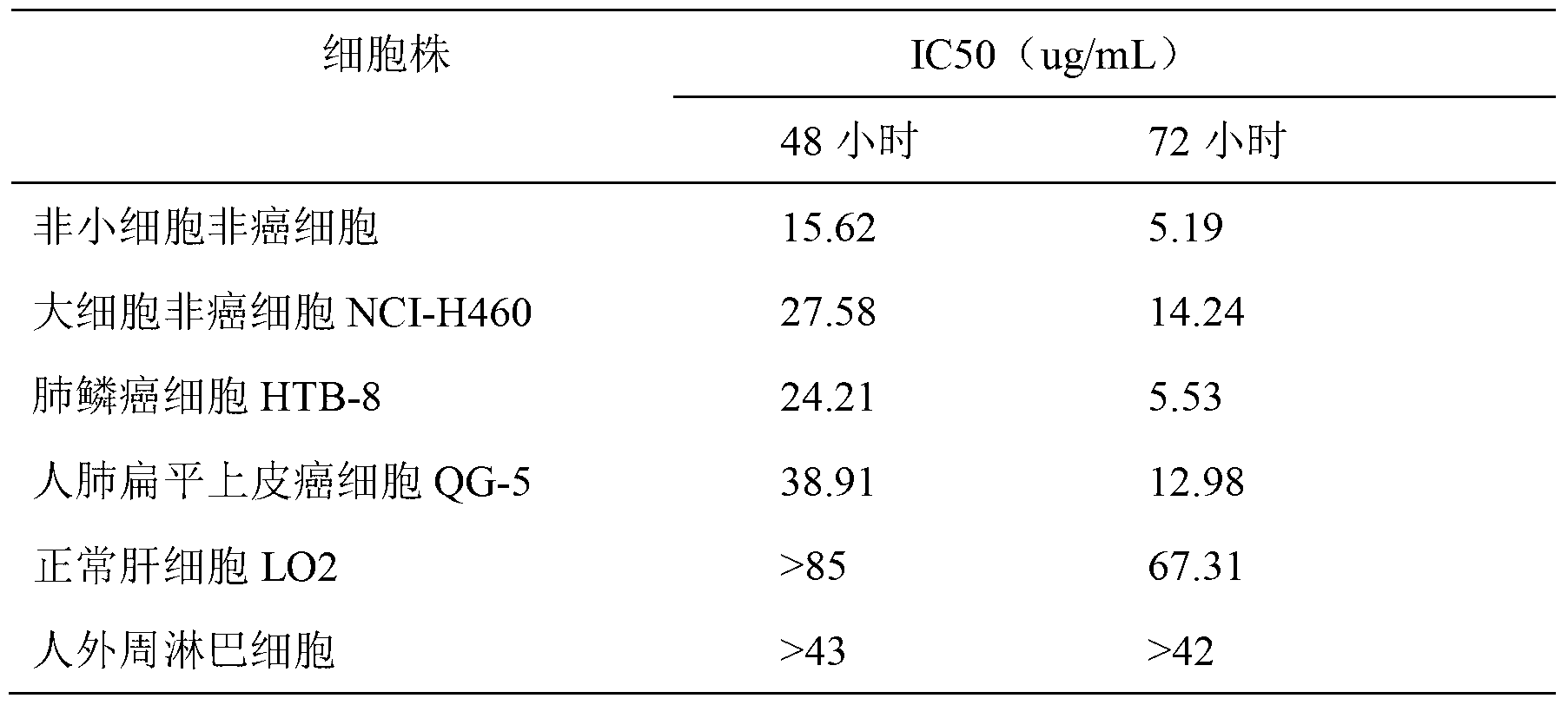
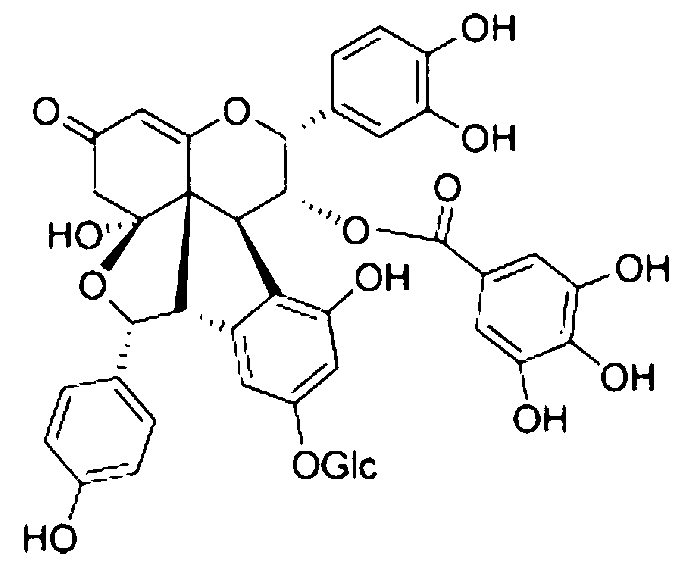
The invention discloses application of Aphanamixoid A to lung cancer treatment medicine and belongs to the technical field of novel application of medicine. The compound has good lung cancer resistance activity and can be used for preparing the lung cancer resistance medicine. The Aphanamixoid A can significantly inhibit non-small cell lung cancer cells A 549, giant-cell lung cancer cells NCI-H460, lung squamous carcinoma cells HTB-8 and human lung pavement epithelium cancer cells QG-5, wherein the non-small cell lung cancer cells A 549, the giant-cell lung cancer cells NCI-H460, the lung squamous carcinoma cells HTB-8 and the human lung pavement epithelium cancer cells QG-5 are cultured in vitro; the inhibiting effect of the Aphanamixoid A on normal human hepatic cells LO2 and peripheral blood lymphocyte is small, and therefore obvious selectivity is achieved. The application of the Aphanamixoid A to preparation of the lung cancer treatment medicine is made public for the first time. The skeleton type is fully novel, and inhibitory activity to the lung cancer cells is surprisingly strong.
Owner:吴俊华
Human big cell lung cancer cell strain with high osseous metastasis potency
InactiveCN103131669BHigh bone metastatic potentialMicrobiological testing/measurementMicroorganism based processesIndividual animalLung cancer cell line
The invention belongs to the field of biotechnology and microorganism animal cell line and particularly relates to a human big cell lung cancer cell strain with high osseous metastasis potency and a purpose of the human big cell lung cancer cell strain with high osseous metastasis potency. A human big cell lung cancer parental generation cell NCI-H460 is utilized as a source cell for screening an osseous metastasis cell, the human big cell lung cancer cell line H460BM with high osseous metastasis potency is screened, cultivated and built by screening six times inside and outside an immunodeficient mice. A preservation number is CGMCC No. 5406. The cell line can be used as an ideal cell model of directional osseous metastasis research of human lung cancer and for transformation mechanism research and anti-metastasis drug screening of human cancer.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Application of compound helicascolide A to preparation of anti-tumor medicines
The invention discloses application of a compound helicascolide A to preparation of anti-tumor medicines. The compound helicascolide A has quite strong cytotoxic activity for glioma cells SF-268, breast cancer cells MCF-7, large-cell lung cancer cells NCI-H460 and human liver cancer cells HepG-2. It is shown that the compound helicascolide A has good anti-tumor activity, and can be applied to preparation of the anti-tumor medicines. Thus, candidate medicines are provided for the research and development of new anti-tumor medicines, and the scientific basis is provided for the development and utilization of natural active substances from plant endophytic fungi.
Owner:东岱(济南)智能技术有限公司
Compound Chinese actinidia root Chinese medicinal composition and preparation method and application thereof
InactiveCN102204974BHas a tumor suppressive effectAntineoplastic agentsPlant ingredientsActinidiaHep G2
The invention discloses a compound Chinese actinidia root Chinese medicinal composition and a preparation method and application thereof, belonging to the technical field of traditional Chinese medicines. The Chinese medicinal composition is characterized by comprising the following traditional Chinese medicines in parts by weight: 40-80 parts of Chinese actinidia root, 20-40 parts of salvia chinensis, 20-40 parts of herba scutellariae barbatae, 20-40 parts of herba oldenlandiae and 20-40 parts of giant knotweed. The compound Chinese actinidia root Chinese medicinal composition has remarkableproliferation inhibition effects on various cancer cells such as a hepatoma cell line Hep-G2, a lung cancer cell line NCI-H460, a gastric cancer cell line MGC-803, a breast cancer cell line MCF-7, a colon cancer cell line HCT-116 and the like, and can be used for reducing physical and psychological pains of a cancer patient in the chemo-treatment process and solving the problems of difficult and expensive administration.
Owner:ZHEJIANG SIXIAN PHARMA
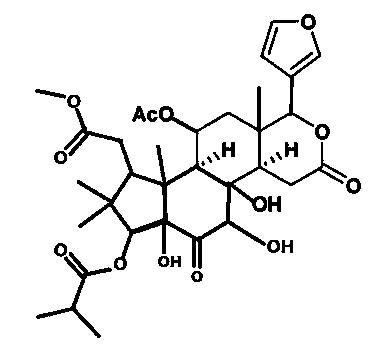
Application of Lycojaponicumin A in medicaments for treating lung cancer
ActiveCN103463043AStrong cytotoxicityHighlight substantive featuresOrganic active ingredientsRespiratory disorderOncologyNon-small cell lung cancer (NSCLC)
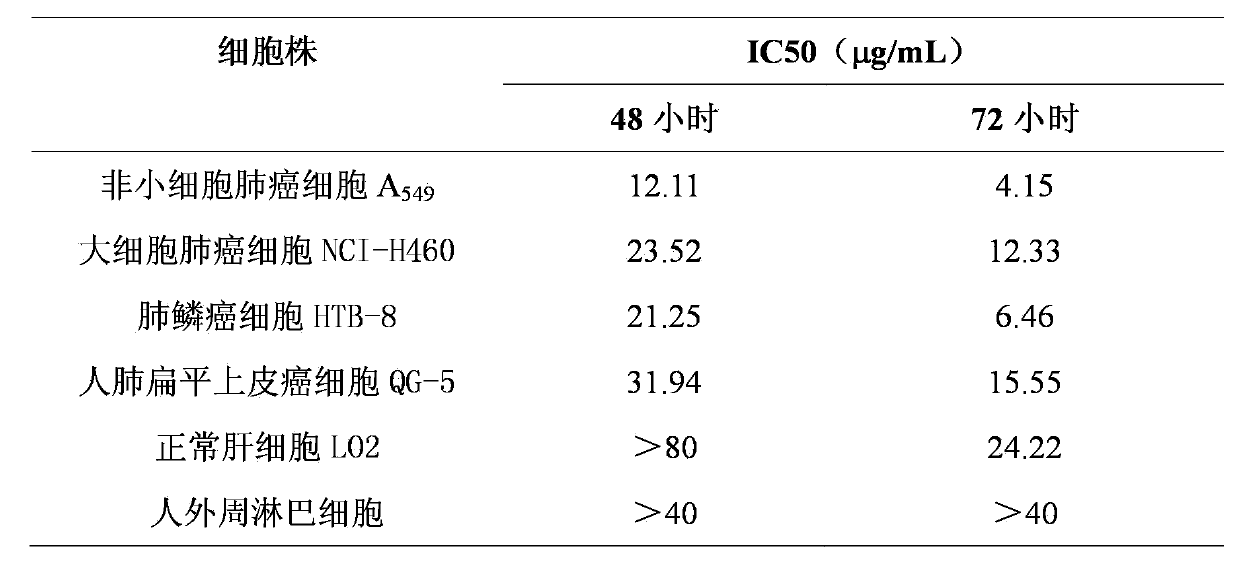
The invention discloses application of Lycojaponicumin A in preparation of medicaments for treating lung cancer. The compound shows good anti-lung cancer activity, can be used for preparing anti-lung cancer medicaments, and belongs to the technical field of new application of medicaments. The Lycojaponicumin A can prominently inhibit non-small cell lung cancer cells A549, large cell lung cancer cells NCI-H460, lung squamous cancer cells HTB-8 and human lung flat epithelial cancer cells QG-5 cultured in vitro, has a low inhibiting effect on human normal liver cells LO2 and peripheral blood lymphocytes, and shows obvious selectivity. The application of the Lycojaponicumin A in preparation of the medicaments for treating the lung cancer is made public for the first time; and because the skeleton type is completely novel, the Lycojaponicumin A has an unexpectedly strong lung cancer cell inhibiting activity.
Owner:安徽创意达技术转移服务有限公司
Acridine-1,2,4-triazole-5-thione compound and its preparation method and application
ActiveCN104277028BIncrease the conjugate areaEasy to embedOrganic active ingredientsOrganic chemistrySodium sulfocyanateFiltration
The invention discloses an acridine-1,2,4-triazole-5-thioketone compound and a preparation method and applications of the acridine-1,2,4-triazole-5-thioketone compound. The preparation method of the compound comprises the following steps: 1) by taking an o-bromobenzoic acid and p-methoxyaniline as raw materials, taking potassium carbonate and copper powder as catalysts, and taking isopentyl alcohol or n-amyl alcohol as a solvent, reacting so as to obtain a compound 1; 2) carrying out cyclization on the compound 1 by using phosphorus oxychloride so as to obtain a compound 2; 3) after the compound 2 is dissolved by using an organic solvent, in the presence of tetrabutylammonium bromide, reacting the dissolved compound 2 with sodium sulfocyanate so as to obtain a compound 3; 4) after the compound 3 is dissolved by using an organic solvent, reacting the dissolved compound 3 with m-nitrobenzoylhydrazine so as to obtain a compound 4; and 5) reacting the compound 4 with sodium carbonate, carrying out suction filtration on a reactant, collecting filter liquor, adjusting the pH value of the filter liquor to be less than 4, separating out precipitates, and carrying out suction filtration on the precipitates. In-vitro antitumor test results show that the compound has a significant in-vitro antitumor activity to tested MGC80-3, NCI-H460 and T24.
Owner:广西新桂环保科技集团有限公司
Application of Lycojaponicumin B in medicine for treating lung cancer
InactiveCN103463025AStrong cytotoxicityHighlight substantive featuresOrganic active ingredientsAntineoplastic agentsEpitheliumCancer cell
The invention discloses an application of Lycojaponicumin B in preparation of a medicine for treating lung cancer. The compound, namely the Lycojaponicumin B shows high lung cancer resisting activity, can be used for preparing a lung cancer resisting medicine and belongs to the technical field of new applications of medicines. The Lycojaponicumin B can obviously inhibit the non-small-cell lung cancer cell A549, maxicell lung cancer cell NCI-H460, squamous cell lung cancer cell HTB-8 and human lung squamous epithelium cancer cell QG-5 cultured in vitro while realizing a relatively low effect on inhibiting the human normal liver cell LO2 and the peripheral blood lymphocyte cell, thereby showing obvious selectivity. The application of the Lycojaponicumin B in preparation of the medicine for treating lung cancer is disclosed for the first time; the skeleton type of the Lycojaponicumin B belongs to a brand new skeleton type, and the inhibitory activity of the Lycojaponicumin B on lung cancer cells is unexpectedly strong.
Owner:北海市韩能生物科技有限公司
Application of Chukrasone A in preparation of medicaments for treating lung cancer
ActiveCN103432142AStrong cytotoxicityHighlight substantive featuresOrganic active ingredientsAntineoplastic agentsEpidermoid carcinomaHepatocyte
The invention discloses application of Chukrasone A in preparation of medicaments for treating lung cancer. The compound shows excellent activity for resisting lung cancer, can be used for preparing medicaments for resisting lung cancer, and belongs to the technical field of novel application of medicaments. Chukrasone A can remarkably inhibit in vitro cultured non-small cell lung cancer cells A549, large cell lung cancer cells NCI-H460, squamous cell lung carcinoma cells HTB-8 and human lung epidermoid carcinoma QG-5, but hardly has inhibition effects on human normal hepatocytes LO2 and peripheral blood lymphocytes, and shows a remarkable selectivity. The application of Chukrasone A in preparation of medicaments for treating lung caner is disclosed for the first time, and the frame type belongs to a brand-new frame type, so that the inhibition activity of the Chukrasone A to lung cancer cells is unexpectedly strong.
Owner:NANJING UNIV
Application of lycojaponicumin A in preparation of medicine for treating lung cancer
The invention discloses application of Lycojaponicumin A in preparation of medicaments for treating lung cancer. The compound shows good anti-lung cancer activity, can be used for preparing anti-lung cancer medicaments, and belongs to the technical field of new application of medicaments. The Lycojaponicumin A can prominently inhibit non-small cell lung cancer cells A549, large cell lung cancer cells NCI-H460, lung squamous cancer cells HTB-8 and human lung flat epithelial cancer cells QG-5 cultured in vitro, has a low inhibiting effect on human normal liver cells LO2 and peripheral blood lymphocytes, and shows obvious selectivity. The application of the Lycojaponicumin A in preparation of the medicaments for treating the lung cancer is made public for the first time; and because the skeleton type is completely novel, the Lycojaponicumin A has an unexpectedly strong lung cancer cell inhibiting activity.
Owner:安徽创意达技术转移服务有限公司
Application of lycojaponicumin C in the preparation of drugs for treating lung cancer
ActiveCN103463068BOrganic active ingredientsAntineoplastic agentsEpidermoid carcinomaCancer research
The invention discloses an application of Lycojaponicumin C in the preparation of medicines for treating lung cancer. The compound shows good anti-lung cancer activity, can be used for the preparation of anti-lung cancer medicines and belongs to the technical field of new applications of medicines. The Lycojaponicumin C can be used for obviously inhibiting a non-small cell lung cancer cell A549, a large-cell lung cancer cell NCI-H460, a lung squamous carcinoma cell HTB-8 and a human lung epidermoid carcinoma cell QG-5 which are cultured in vitro, has a low inhibition effect on a human normal lung cell LO2 and a peripheral blood lymphocyte and shows up obvious selectivity. The application of the Lycojaponicumin C in the preparation of the medicines for treating the lung cancer is disclosed for the first time. As the skeleton type of the Lycojaponicumin C belongs to a brand-new skeleton type, the Lycojaponicumin C has unexpected strong inhibitory activity on the lung cancer cells.
Owner:安徽网萌科技发展股份有限公司
A kind of platinum (ii) complex synthetic method and application of 6-aminooxidized isoapomorphine
InactiveCN103524564BSignificant in vitro antitumor activityGood potential medicinal valueOrganic active ingredientsGroup 8/9/10/18 element organic compoundsChemical structureHuman tumor
The invention discloses a new platinum (II) complex of 6-amino oxoisoaporphine, namely monochloro.dimethyl sulfoxide.6-amino oxoisoaporphine platinum (II), and a synthesis method and an application thereof. The complex is synthesized by the steps of dissolving 6-amino oxoisoaporphine and dichloro.di(dimethyl sulfoxide) platinum (II) in a polar solvent, and heating or carrying out a reflux reaction to obtain the target product; specifically the complex can be synthesized by a solution method, and can also be synthesized by a solvothermal method. Through investigation of proliferation inhibitory activity of the complex against HepG2, BEL-7404 and NCI-H460 and other human tumor cell strains, the complex is found to have significant in-vitro antitumor activity against the 3 kinds of tumor strains and have relatively good potential medicinal value, and is expected to be used in preparation of various antitumor medicines. The complex has the chemical structure represented by the following formula as described in the specification.
Owner:GUANGXI NORMAL UNIV
Application of polyflavanostilbene A in preparation of medicaments for treating lung cancer
InactiveCN103263430AStrong cytotoxicityHighlight substantive featuresOrganic active ingredientsAntineoplastic agentsPeripheral blood lymphocyteInhibitory effect
The invention discloses an application of polyflavanostilbene A in preparation of medicaments for treating lung cancer. The compound shows good anti-lung cancer activity, can be used for preparing anti-lung cancer medicaments and belongs to the technical field of new uses of the medicaments. The polyflavanostilbene A can significantly inhibit non-small cell lung cancer cells A549, large cell lung cancer cells NCI-H460, lung squamous carcinoma cells HTB-8 and human lung squamous cell carcinoma cells QG-5, which are cultured in vitro, realize a smaller inhibition effect against human normal liver cells LO2 and peripheral blood lymphocytes and show obvious selectivity. The use of the polyflavanostilbene A in the preparation of the medicaments for treating the lung cancer, disclosed by the invention, belongs to the first disclosure, the framework type belongs to the brand new framework type, and the strong inhibition activity against the cells of the lung cancer is further unexpected.
Owner:NANJING ZHENGLIANG MEDICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Phthalizine [1,2,b] quinazoline-8-ketone compound and preparation method and application in antitumor drugs of phthalizine [1,2,b] quinazoline-8-ketone compound Phthalizine [1,2,b] quinazoline-8-ketone compound and preparation method and application in antitumor drugs of phthalizine [1,2,b] quinazoline-8-ketone compound](https://images-eureka.patsnap.com/patent_img/ff86e77d-5670-414a-8b2a-a33221d46931/BDA0000886530140000011.PNG)
![Phthalizine [1,2,b] quinazoline-8-ketone compound and preparation method and application in antitumor drugs of phthalizine [1,2,b] quinazoline-8-ketone compound Phthalizine [1,2,b] quinazoline-8-ketone compound and preparation method and application in antitumor drugs of phthalizine [1,2,b] quinazoline-8-ketone compound](https://images-eureka.patsnap.com/patent_img/ff86e77d-5670-414a-8b2a-a33221d46931/BDA0000886530140000021.PNG)
![Phthalizine [1,2,b] quinazoline-8-ketone compound and preparation method and application in antitumor drugs of phthalizine [1,2,b] quinazoline-8-ketone compound Phthalizine [1,2,b] quinazoline-8-ketone compound and preparation method and application in antitumor drugs of phthalizine [1,2,b] quinazoline-8-ketone compound](https://images-eureka.patsnap.com/patent_img/ff86e77d-5670-414a-8b2a-a33221d46931/BDA0000886530140000041.PNG)