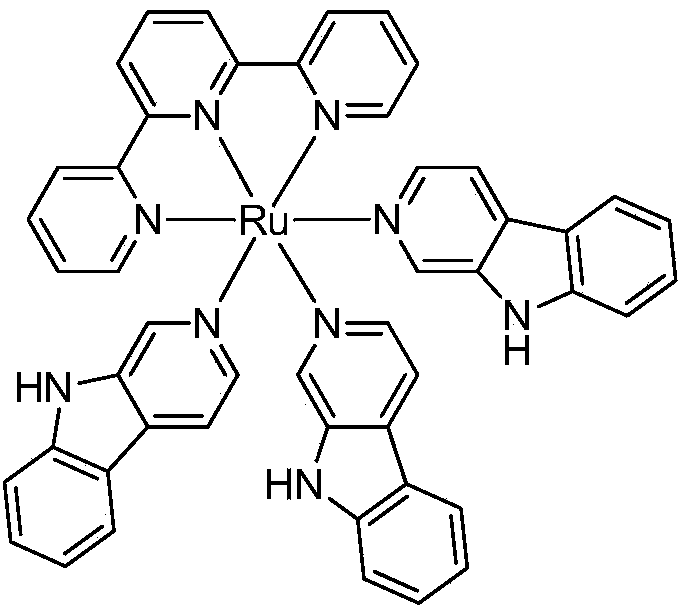
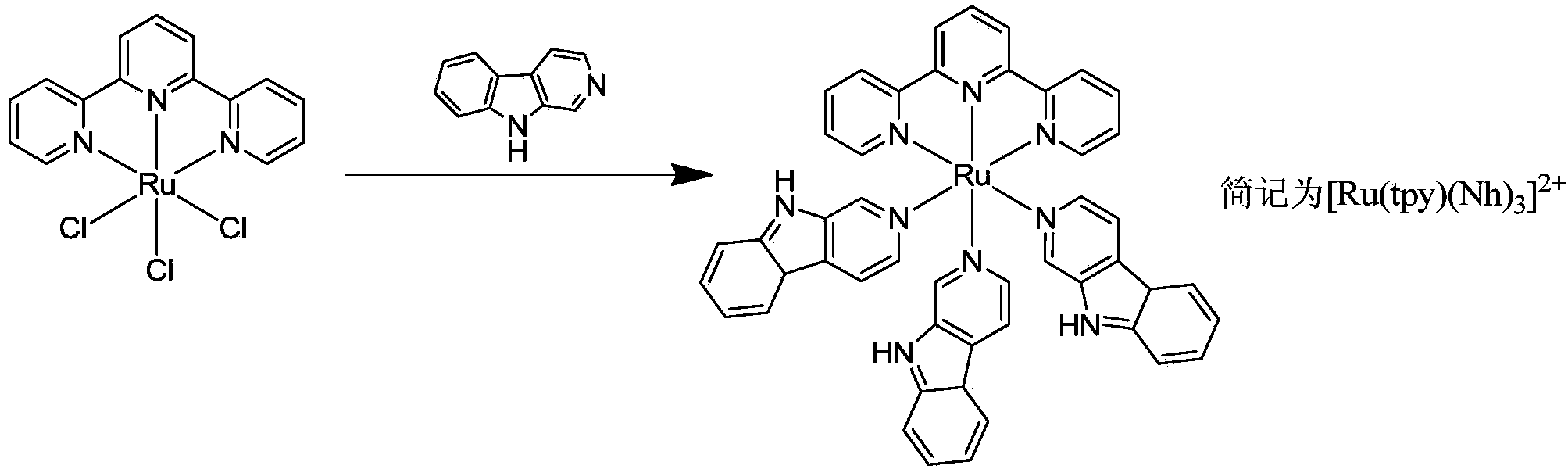
Norharman-ruthenium (II) polypyridine complex with antitumour activity
A technology of anti-tumor activity and anti-tumor drugs, applied in the field of new Norharman-ruthenium polypyridine complexes, to achieve the effects of inhibiting tumor proliferation, enhancing cell transmembrane ability, and stabilizing molecular structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Complex [Ru(tpy)(Norharman) 3 ]·(CF 3 SO 3 ) 2 Synthetic method:
[0022] (1)Ru(tpy)Cl 3 Synthesis
[0023] The synthesis of this compound can be prepared by referring to existing literature (B.P.Sullivan, D.J.Salmn and T.J.Meyer, Inorg.Chem., 1978, 17, 3334). Weigh RuCl 3 ·nH 2 O2.62g (about 10mmol), 2,2':6',2''-terpyridine 2.33g (10mmol), at 250cm 3 In absolute ethanol, heat to reflux for about 3 hours. After cooling to room temperature, filter with suction, wash the precipitate with ice water and cold acetone, and dry in vacuo to obtain purple-black microcrystals. The average yield is 80%.
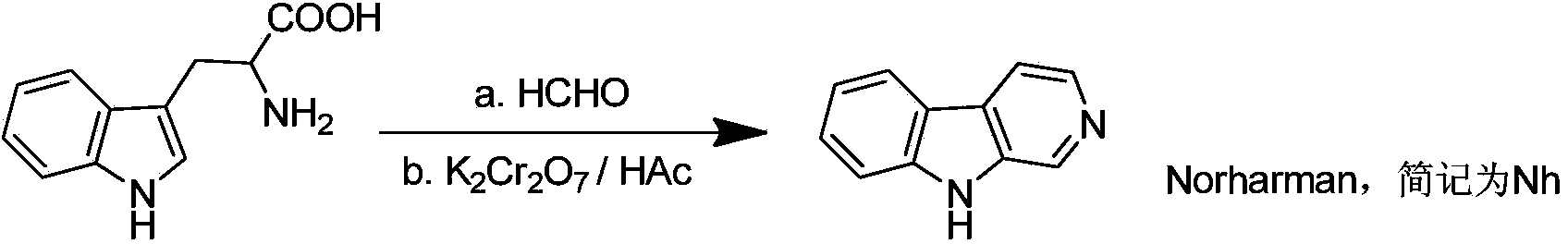
[0024] (2) Synthesis of Norharman
[0025]The synthesis of this compound can be prepared by referring to existing literature (H.R.Snyder, H.G.Walker and F.X.Werber.J.Am.Soc.Chem., 1949, 71, 527-529). Weigh 10.0g (about 49mmol) of L-tryptophan and 4.0g (about 100mmol) of sodium hydroxide in 500cm 3 In ultrapure water, stir until completely dissolved. Then add 40% met...
Embodiment 2
[0029] [Ru(tpy)(Norharman) 3 ]·(CF 3 SO 3 ) 2 The confocal laser experiment of HeLa cells and complexes is taken as an example in this example.
[0030] Cell culture: HeLa cells were cultured in DMEM medium containing 10% fetal bovine serum, cells (5×l0 8 / L) inoculated in a special glass bottom petri dish for confocal microscopy, the diameter of the petri dish is 35mm, the thickness of the cover glass is 0.085-0.13mm, the diameter of the micropore in the center of the petri dish is 10mm, and 5% CO 2 and 95% air conditions, cultured at 37°C, and grown adherently for 24 hours.
[0031] Confocal Microscopy-Cell Imaging: HeLa cells with [Ru(tpy) (Norharman) 3 ]·(CF 3 SO 3 ) 2 (5μM) incubate for 30min, 1h, 1.5h and 2h, aspirate the culture solution, and then wash with PBS buffer for 3-4 times, image on a Leica TCS SP5 laser scanning confocal microscope, use a 63× / 1.4 oil lens, and use a 350nm Light is used as an excitation light source to collect fluorescence in the range...
Embodiment 3
[0033] [Ru(tpy)(Norharman) 3 ]·(CF 3 SO 3 ) 2 In this example, the ICP-MS experiment of HeLa cells and complexes is taken as an example.
[0034] Cell culture: HeLa cells were cultured in DMEM medium containing 10% fetal bovine serum, cells (5×l0 8 / L) inoculated in a cell culture dish with a diameter of 100 mm and 5% CO 2 and 95% air conditions, cultured at 37°C, and grown adherently for 24 hours.
[0035] Inductively coupled plasma mass spectrometry (ICP-MS): HeLa cells and [Ru(tpy) (Norharman) 3 ]·(CF 3 SO 3 ) 2 (5μM) incubate for 30min, 1h, 1.5h and 2h, suck out the culture medium, and then wash with PBS buffer 3 to 4 times. Digest into single cells with 0.25% trypsin and divide the cells into 4 parts. The first was used for cell counting, the second was used to isolate the cytoplasm using the Thermo's Cytoplasm Isolation Kit, and the third was used to isolate the nuclei using the Thermo's Nuclei Isolation Kit. Disperse the fourth cell, the isolated cytoplasm an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com