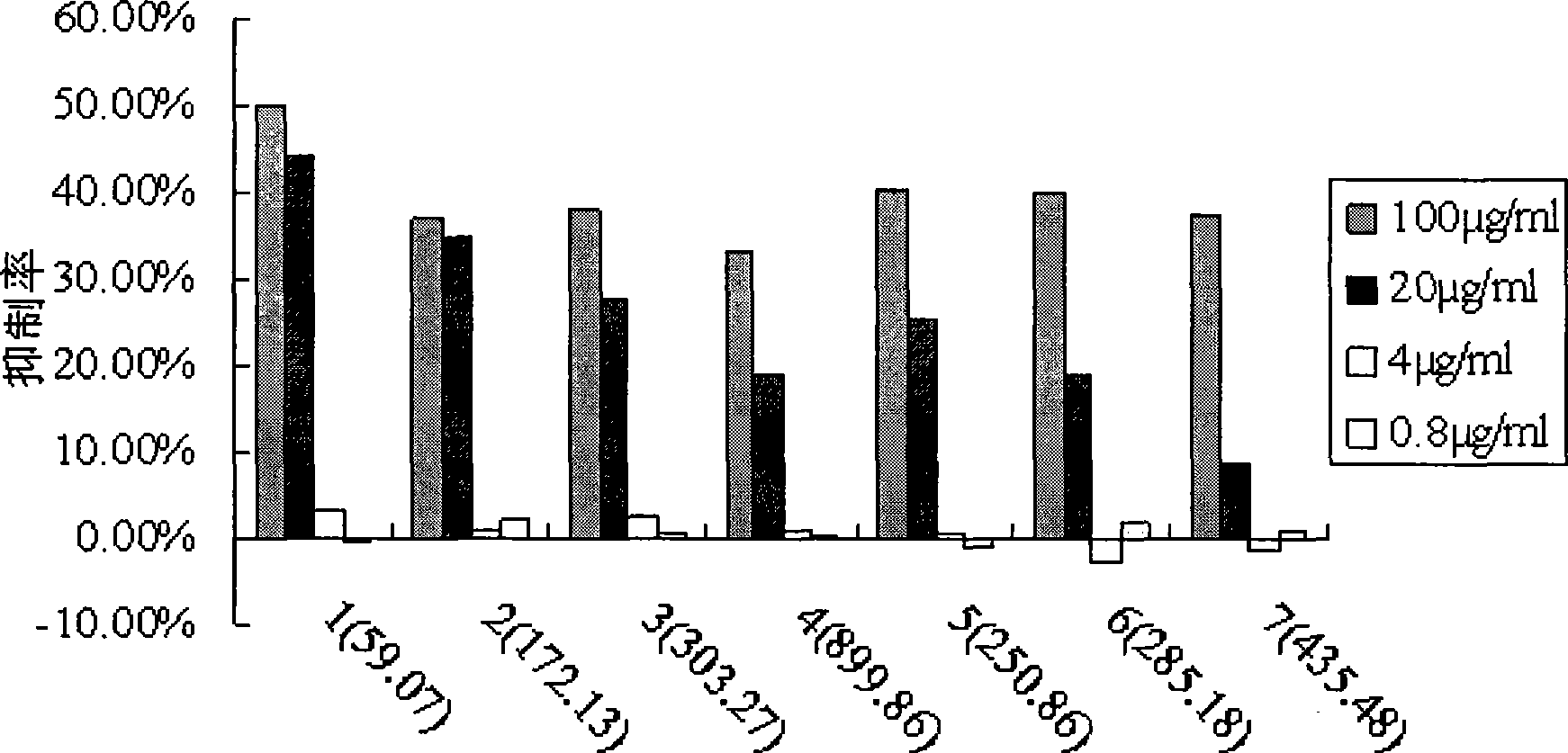Shikonin carbohydrate derivatives and synthetic method and use thereof
The technology of a carbohydrate derivative and a synthesis method is applied in synthesis and its application in tumor suppression, and can solve problems such as side effects and obvious morphine addiction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0032] Example 1: Shicaoning preparation method
[0033] Take the bruised tissue culture solution of Lilacia yunnanensis cultivated in the dark, and extract it three times with petroleum ether with a boiling range of 60-90°C until it becomes colorless. The extracts were combined, and the solvent was concentrated under reduced pressure to obtain a black-red paste, which was dissolved by adding chloroform, and an appropriate amount of silica gel was added, and the solvent was evaporated to dryness, followed by column chromatography. The chromatographic column is packed with 200-300 mesh silica gel, and the column chromatography is carried out with ethyl acetate:petroleum ether=1:7 to obtain pure shikonin.
example 2
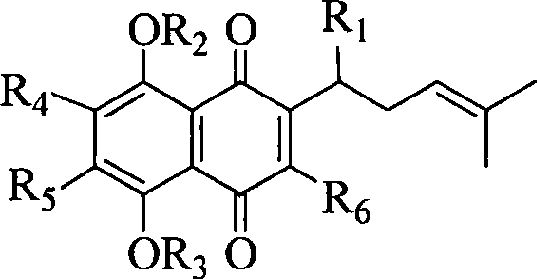
[0034] Example two: the synthetic method of formula I class shikonin derivatives
[0035] Take 0.5mmol of shikonin and 0.4mmol of fully acetylated mercapto sugar compound and dissolve them in 20ml of ethanol solution, stir and react at room temperature at 0°C to 30°C until the point of sugar compound disappears, followed by TLC detection. Add an appropriate amount of silica gel to concentrate the solvent under reduced pressure, and perform column chromatography with ethyl acetate:petroleum ether=1:2 to obtain the glycosyl shikonin derivative with a yield of about 85%.
[0036] The reaction takes glucose as an example:
[0037]
[0038] Compound 1
[0039] Compounds 2-7 can be obtained in the same way.
[0040]
[0041] Compound 2 Compound 3 Compound 4 Compound 5
[0042]
[0043] Compound 6 Compound 7
example 3
[0044] Example 3: Synthesis of Formula I Deprotected Glycosyl Shikonin Derivatives
[0045] Dissolve 0.1mmol of compound 1 in 5ml of acetonitrile, add an appropriate amount of concentrated ammonia water dropwise at a temperature ranging from -4°C to 5°C, react until the raw material point disappears, neutralize it with dilute hydrochloric acid, add 0.05g of silica gel to the product and evaporate it to dryness naturally, then wash with ethanol : Chloroform=1:5 column chromatography, the product was obtained, and the yield was 90%.
[0046] The reaction takes glucose as an example:
[0047]
[0048] Compound 8
[0049] Compounds 9-14 can be obtained by the same method.
[0050]
[0051] Compound 9 Compound 10 Compound 11 Compound 12
[0052]
[0053] Compound 13 Compound 14
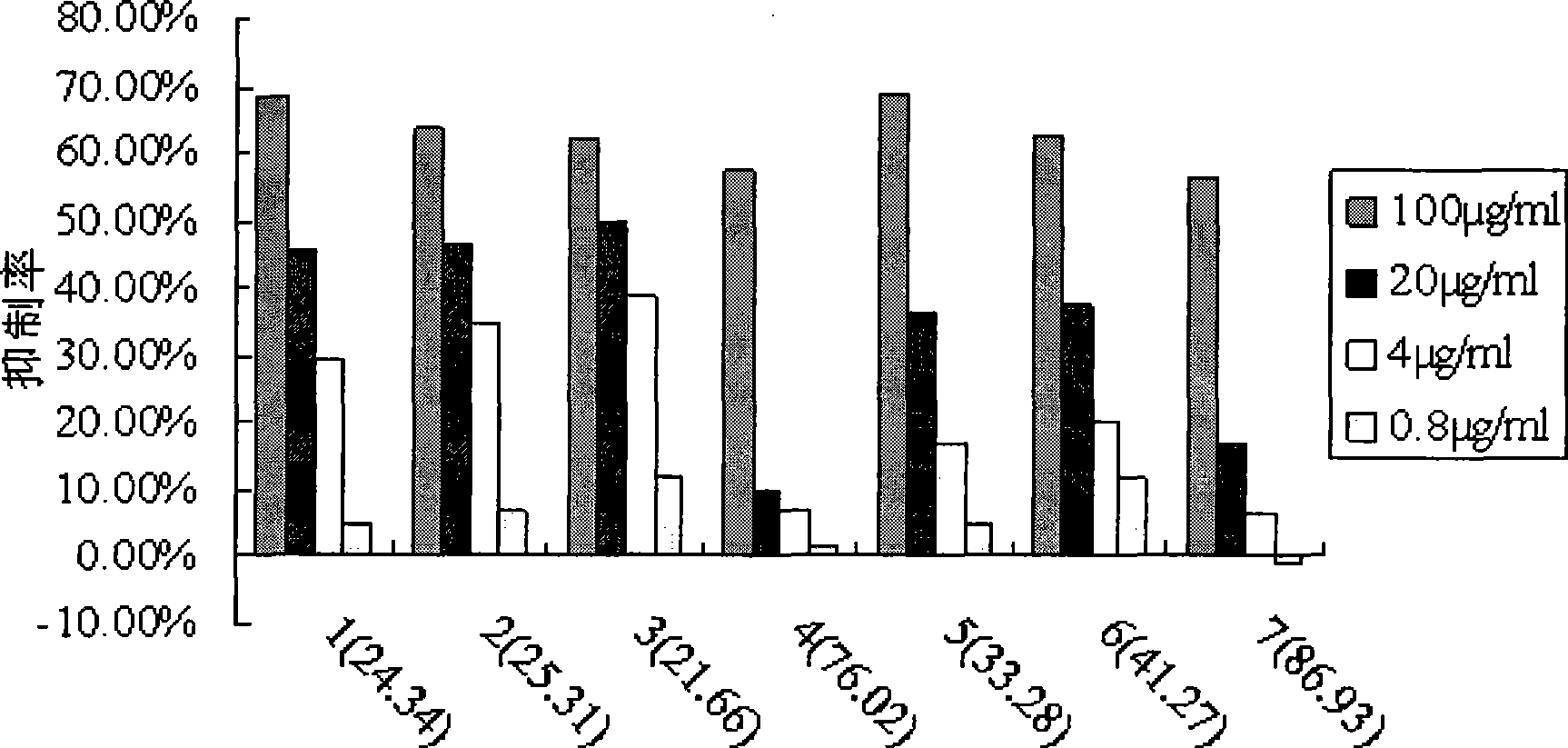
[0054] Example 4. Application of Formula I Shikonin Saccharide Derivatives
[0055] We studied the anti-tumor activity of Shikonin sugar derivatives of formul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com