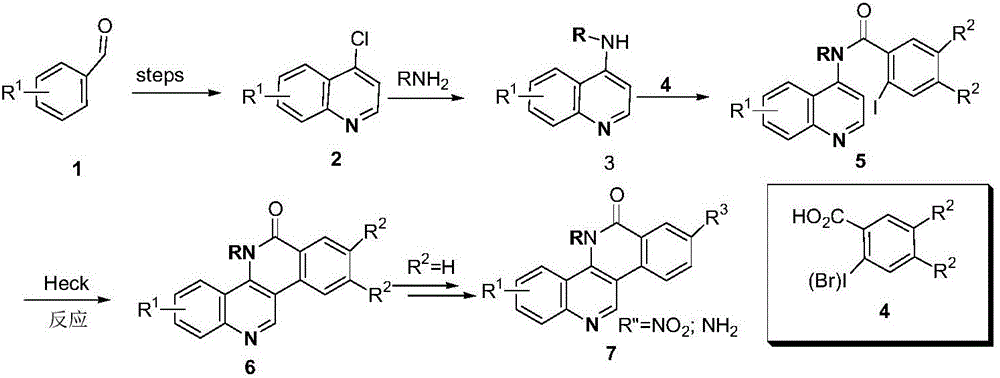
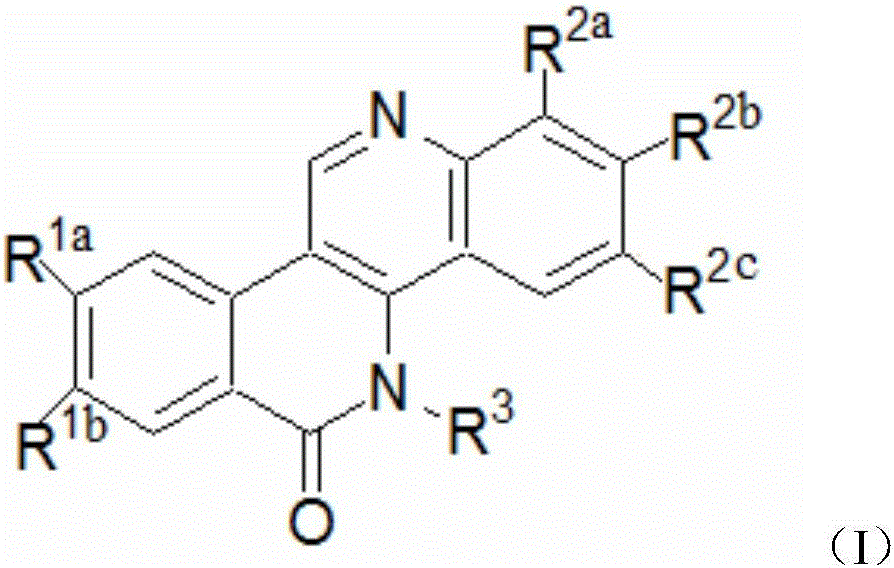
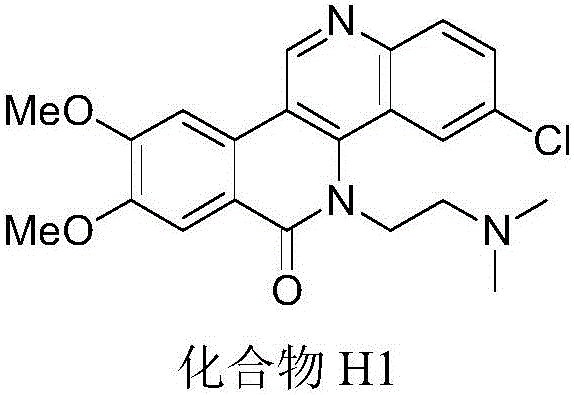
Dibenzonaphthyridinone compounds, preparation method, and applications thereof
A kind of technology of benzonaphthyridone and compound, applied in the field of antitumor compounds, can solve the problems such as cumbersome steps, not easy to obtain, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0033] Preparation Example 1: Preparation of Compound H1: 5H-8,9-dimethoxy-5-(2-N,N-dimethylaminoethyl)-3-chlorodibenzo[c,h][1,6]-naphthyridin-6-one
[0034]
[0035] (1) Add 3,4-dimethoxyphenylacetic acid (1.96g, 10mmol), absolute ethanol (5mL), cyclohexane (5mL) and concentrated sulfuric acid (1mL) to a 50mL round bottom flask in turn under stirring , after installing the water separator and straight condenser tube, reflux at 100°C until there is no more water in the water separator; after the reaction is completed, remove cyclohexane and excess ethanol under reduced pressure, and add ethyl acetate to the flask ester (30mL), the mixture was washed twice with ice water, 30mL each time, then washed twice with saturated sodium bicarbonate solution, 30mL each time, extracted with organic solvent for 3 times, the organic layers were combined, and the organic layer was dried with anhydrous magnesium sulfate , filtered, the solvent was removed under reduced pressure, and silica ...
preparation Embodiment 2
[0043] Preparation Example 2: Preparation of Compound H2 5H-8,9-dimethoxy-5-(2-N,N-dimethylaminoethyl)-1-chlorodibenzo[c,h][1,6]-naphthyridin-6-one
[0044]
[0045] Using o-chloroaniline instead of p-chloroaniline, other preparation methods were the same as in Preparation Example 1 to obtain the target compound H2 in the form of white powder.
[0046] Compound H2: Yield 36%, m.p.222~223℃; 1 H NMR (500MHz, CDCl 3 )δ:2.26(s,6H,N(CH 3 ) 2 ), 2.94(t, J=7.10Hz, 2H, CH 2 ),4.05(s,3H,OCH 3 ),4.11(s,3H,OCH 3 ), 4.67(t, J=7.10Hz, 2H, CH 2 ),7.49(dd,J=8.70Hz,7.45Hz,1H,ArH),7.69(s,1H,ArH),7.85(dd,J=7.45Hz,0.95Hz,1H,ArH),7.89(s,1H , ArH), 8.41(d, J=8.70Hz, 1H, ArH), 9.65(s, 1H, CH=N); 13 C NMR (125MHz, CDCl 3)δ:45.89,49.47,56.38,56.43,57.77,102.16,108.87,112.63,119.91,120.13,120.50,123.78,125.55,127.08,129.52,134.52,141.26,144.89,146.09,150.91,154.42,163.89;HRMS(ESI ): m / z calculated value [M+H] + 412.1428 (C 22 h 22 ClN 3 o 3 ), the experimental value is 412.1443.
preparation Embodiment 3
[0047] Preparation Example 3: Preparation of Compound H3 5H-8,9-dimethoxy-5-(2-N,N-dimethylaminoethyl)-2-chlorodibenzo[c,h][1,6]-naphthyridin-6-one
[0048]
[0049] Using m-chloroaniline instead of p-chloroaniline, the other preparation methods were the same as in Preparation Example 1 to obtain the target compound H3 as a white powdery solid.
[0050] Compound H3: Yield 14%, m.p.235~236℃; 1 H NMR (500MHz, CDCl 3 )δ:2.31(s,6H,N(CH 3 ) 2 ),2.99(t,J=7.25Hz,2H,CH 2 ),4.03(s,3H,OCH 3 ),4.10(s,3H,OCH 3 ), 4.61(t, J=7.25Hz, 2H, CH 2 ),7.52(dd,J=9.25Hz,2.30Hz,1H,ArH),7.63(s,1H,ArH),7.84(s,1H,ArH),8.10(d,J=2.30Hz,1H,ArH) ,8.45(d,J=9.25Hz,1H,ArH),9.48(s,1H,CH=N); 13 CNMR (125MHz, CDCl 3 )δ:45.87,49.01,56.38,56.43,57.68,102.02,108.73,111.91,117.32,119.53,126.15,126.75,127.27,129.34,135.06,140.78,146.71,149.32,150.71,154.35,163.59;HRMS(ESI): m / z calculated value [M+H] + 412.1428 (C 22 h 22 ClN 3 o 3 ), the experimental value is 412.1434.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com