Platinum (II) metal complex for specifically inhibiting proliferation of lung carcinoma cell, and synthesis method and application thereof
A synthesis method and compound technology, applied in the field of medicine, can solve the problems of destroying the planarity and independence of the oxidized isoaporphine parent ring, affecting the anti-tumor activity and advantages of the complex, and affecting the active site of the electron cloud density, etc. The effect of good medicinal value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
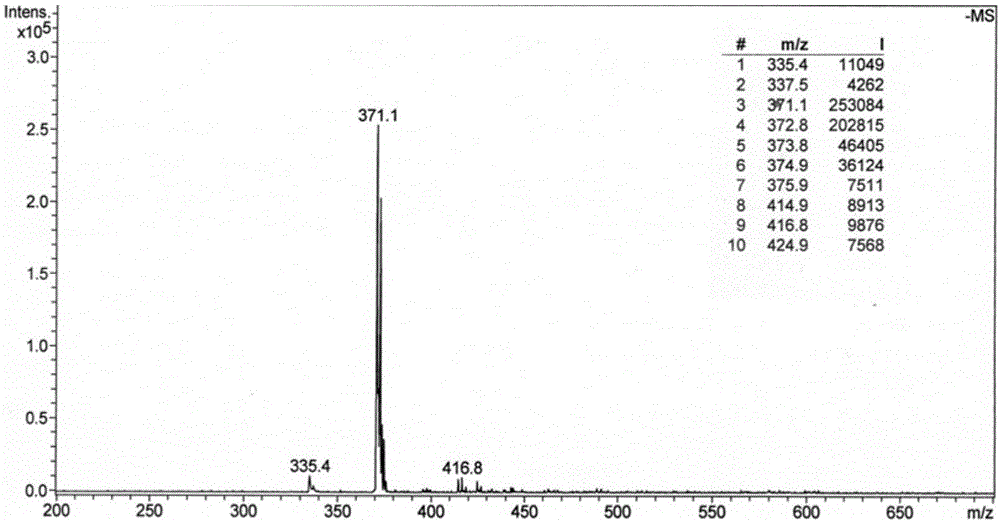
[0038] Example 1: Synthesis of Ligand L, i.e. 9-(3-chloropropanamide)-oxidized isoaporphine
[0039] Ligand L was synthesized according to the following synthetic route:
[0040]
[0041] The specific synthesis method is:
[0042] 1) Preparation of 9-(3-chloropropanamido)-oxidized isoaporphine with reference to existing literature (Tang, H.; etal. Eur. J. Med. Chem., 2009, 44: 2523-2532.) , the detailed steps are as follows:
[0043] 1.1) Dissolve 15.0g (about 0.1mol) of phthalic anhydride and 12.0g (about 0.1mol) of β-phenylethylamine in 100mL of ethanol, reflux for 5 hours, filter while hot, cool to room temperature, precipitate crystals, pump Filtered to obtain white flaky crystal compound a with a yield of 75%. Mix and heat 75g (0.56mol) of anhydrous aluminum trichloride and 15g of sodium chloride to 140°C for melting, slowly add 53g (0.2mol) of compound a to it, after the addition is completed, heat up to 180°C, and stir for 2 hours , introduced into the grinder by...
Embodiment 2
[0061] Embodiment 2: the synthesis of ligand L
[0062] 1) with embodiment 1;
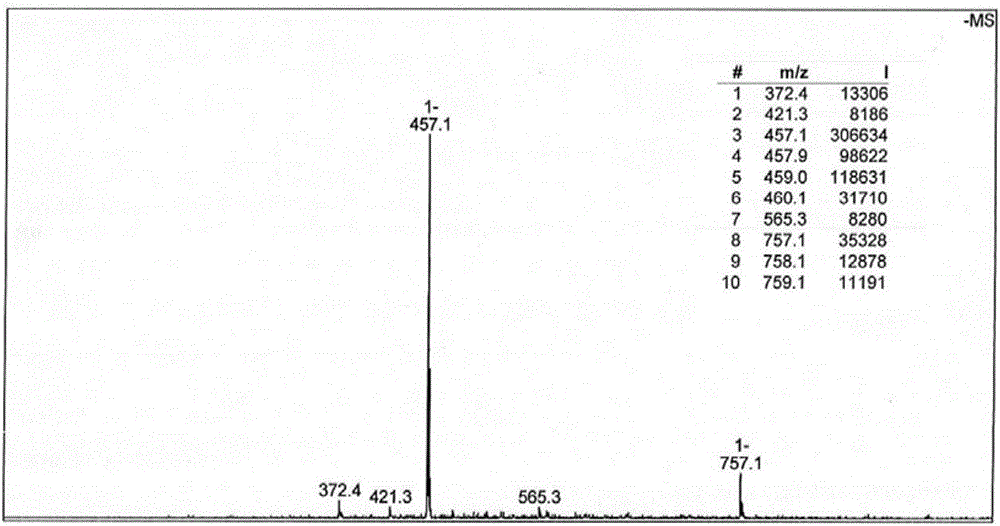
[0063] 2) Weigh 1mmol of 9-(3-chloropropanamide)-oxyisoapophine and 0.8mmol of 2-(2-aminoethyl)pyridine, use ethanol as solvent, the amount of ethanol is 10mL, after dissolving The mixed solution was obtained and reacted at 37°C for 72 hours. The obtained reactant was suction filtered, washed with ether, dried, and then purified by silica gel column chromatography (chloroform / methanol=100:1→10:1, volume ratio) to obtain a yellow solid Product (65% yield).
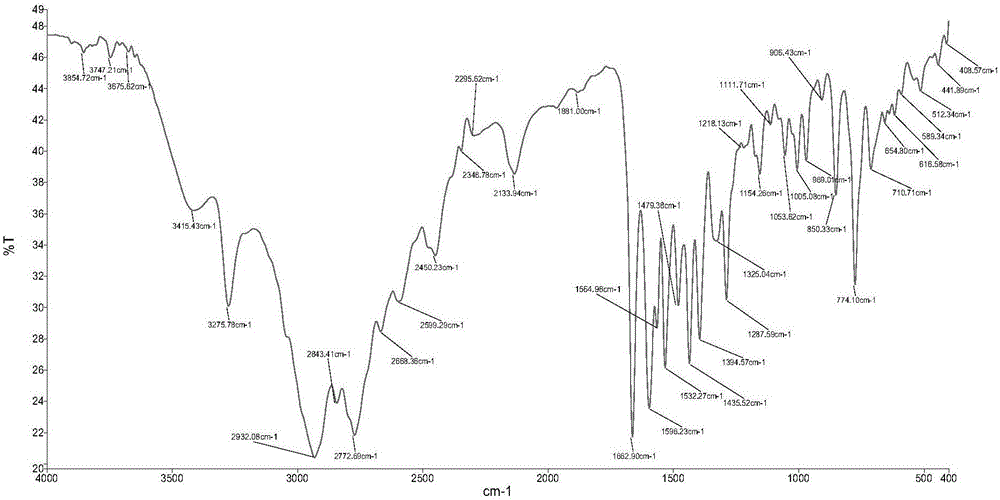
[0064] The obtained product was analyzed by infrared spectrum, high-resolution mass spectrometry, proton nuclear magnetic resonance spectrum, carbon nuclear magnetic resonance spectrum, etc., and determined to be the target product ligand L.
Embodiment 3
[0065] Embodiment 3: the synthesis of ligand L
[0066] 1) with embodiment 1;
[0067] 1) Weigh 1mmol of 9-(3-chloropropanamide)-oxidized isoaporphine and 10mmol of 2-(2-aminoethyl)pyridine, use methanol as solvent, the amount of methanol is about 100mL, after dissolving The mixed solution was obtained and reacted at 90°C for 6 hours. The obtained reactant was suction filtered, washed with ether, dried, and then purified by silica gel column chromatography (chloroform / methanol=50:1→15:1, volume ratio) to obtain a yellow solid Product (80% yield).
[0068] The obtained product was analyzed by infrared spectrum, high-resolution mass spectrometry, proton nuclear magnetic resonance spectrum, carbon nuclear magnetic resonance spectrum, etc., and determined to be the target product ligand L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com