A kind of platinum (ii) complex synthetic method and application of 6-aminooxidized isoapomorphine
A technology of isoaporphine and amino oxidation, applied in the field of medicine, to achieve the effect of good medicinal value and significant in vitro anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Weigh 6-amino-isoaporphine and dichlorobis(dimethylsulfoxide) platinum (II) in the same amount of the same substance, each 1mmol, and dissolve 6-amino-isoaporphine in 45 mL of 40 In% methanol, dissolve dichlorobis(dimethylsulfoxide)platinum(II) in 15mL of water, mix the two solutions, add 2mL of dimethylsulfoxide to the mixture, and react at 65°C for 36 hours After concentrating and evaporating to remove most of the solvent, cooling to room temperature and standing still, a red-brown solid is precipitated, and the solid is separated and dried to obtain a red-brown solid product with a yield of 85%.
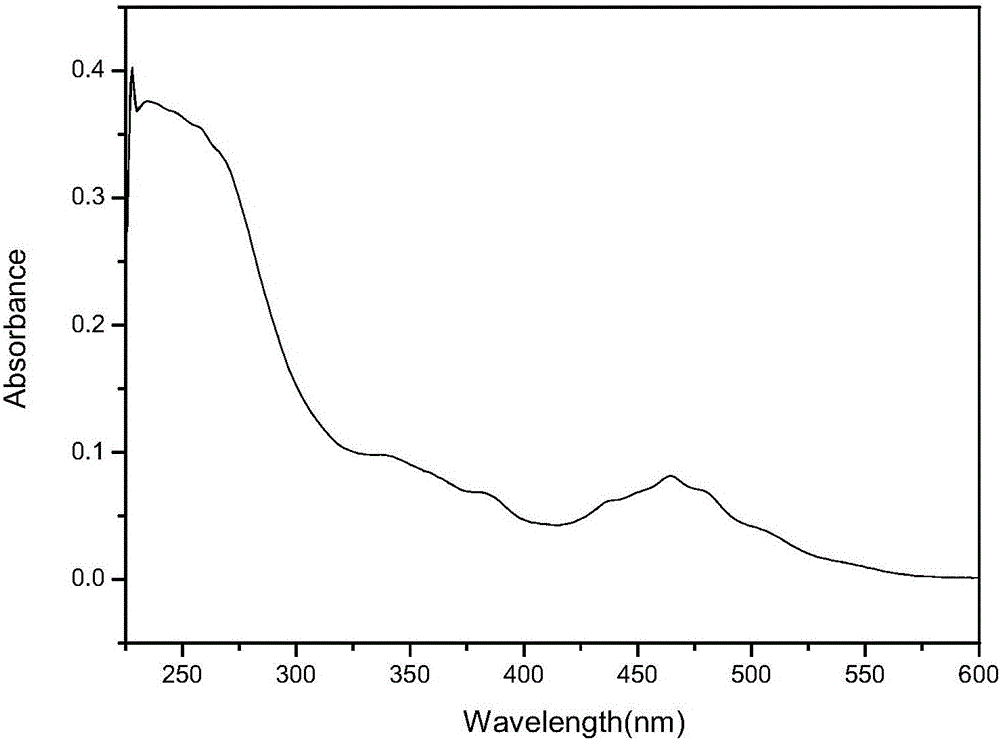
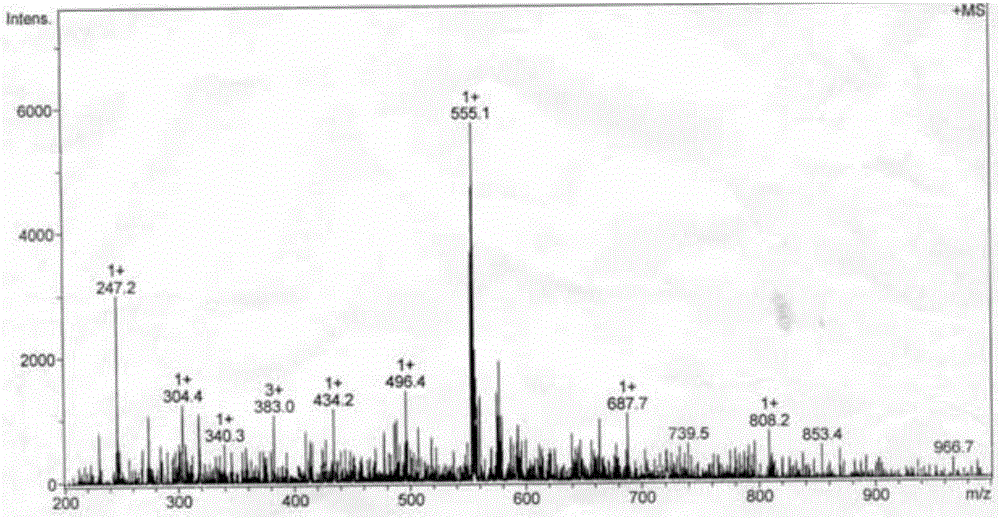
[0041] The obtained red-brown solid product was analyzed by infrared spectroscopy, ultraviolet spectroscopy, electrospray mass spectrometry, and single crystal diffraction analysis. The specific spectral characteristics are as follows:
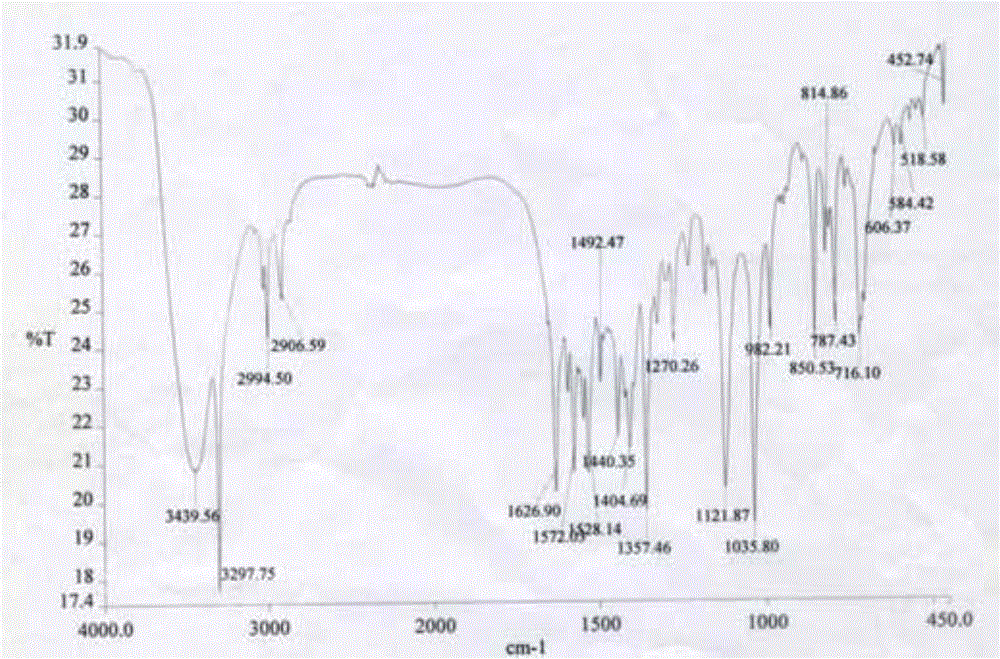
[0042] (1) Infrared spectrum, the spectrum is as figure 1 Shown.
[0043] IR (KBr): 3440, 3298, 2995, 1627, 1572, 1528, 1440, 1405, 1357, 1270, ...
Embodiment 2
[0051] Weigh the same amount of isoaporphine 6-amino oxide and platinum dichlorobis(dimethyl sulfoxide) (II), each 1mmol, dissolve isoaporphine 6-amino oxide in 80 mL of 90 In% ethanol, dissolve dichlorobis(dimethyl sulfoxide) platinum (II) in 40 mL of 40% methanol, mix the two solutions, add 8 mL of dimethyl sulfoxide to the mixture, and hold at 65°C Reacted for 4 hours, concentrated and evaporated to remove most of the solvent, cooled to room temperature, stood still, a reddish-brown solid was precipitated, the solid was separated, and dried to obtain monochlorodimethylsulfoxide·isoaporphinplatin 6-amino oxide (II), the yield is 65%.
Embodiment 3
[0053] Weigh the same amount of isoaporphine 6-amino oxide and platinum dichloro bis(dimethyl sulfoxide) (II), each 1mmol, dissolve isoaporphine 6-amino oxide in 50 mL of 50 In a mixed solution of% methanol and 50% ethanol (volume ratio 1:4), dissolve dichlorobis(dimethylsulfoxide) platinum (II) in 20 mL of a mixed solution of water and methanol (volume ratio 1 :1) In, the two solutions are mixed, and the mixture is reacted at 70°C for 48 hours. After concentrating and evaporating to remove most of the solvent, it is cooled to room temperature and allowed to stand. A reddish-brown solid precipitates. The solid is separated and dried to obtain monochlorine. Dimethyl sulfoxide·6-Amino oxide isoaporphine platinum (II), the yield is 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com