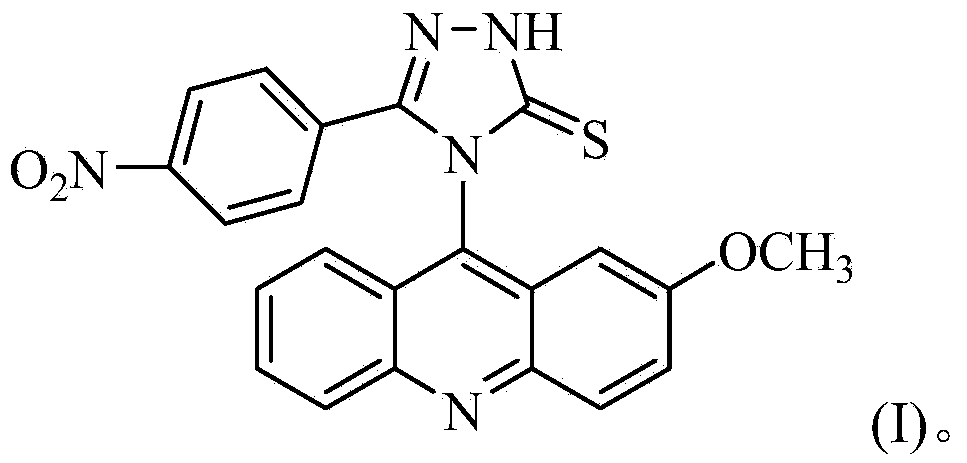
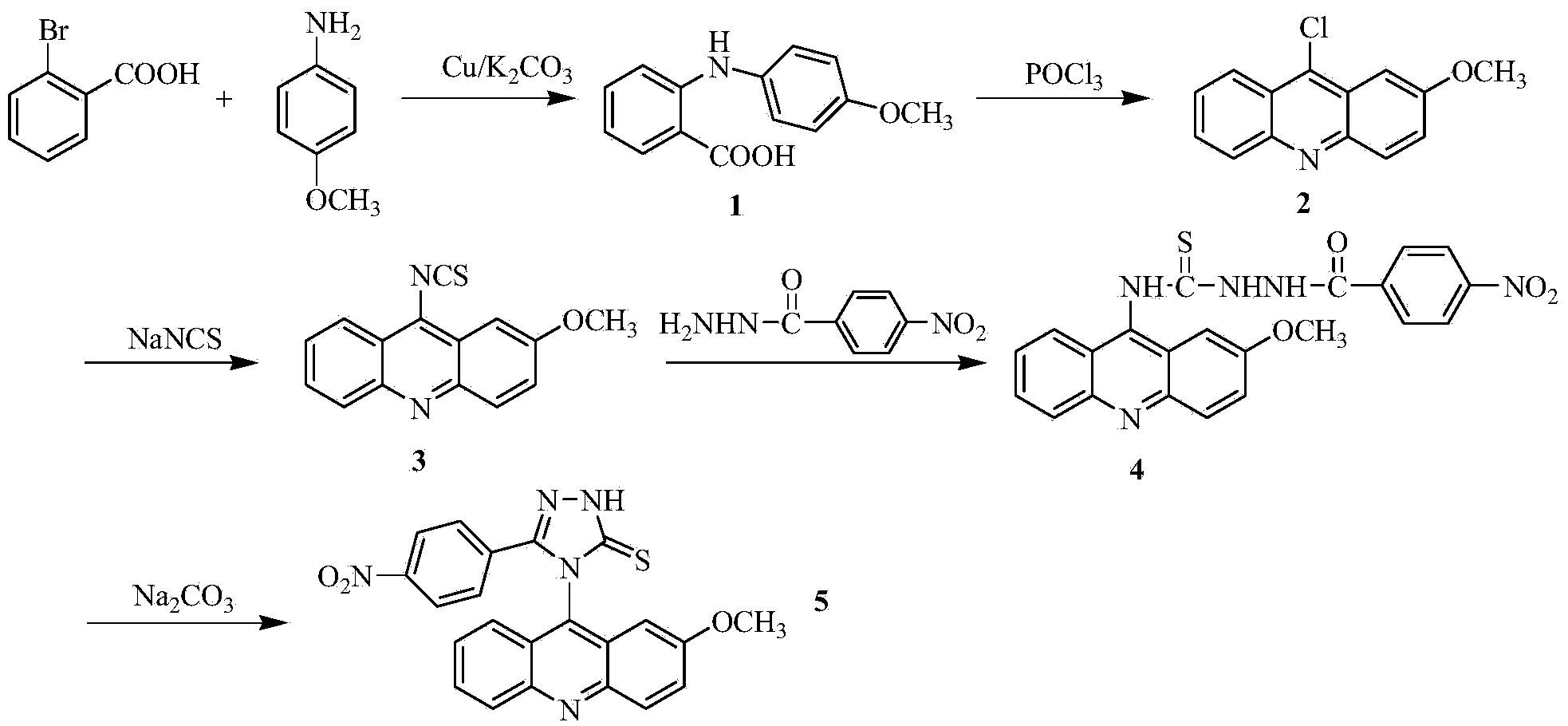
Acridine-1,2,4-triazole-5-thioketone compound and preparation method and applications of acridine-1,2,4-triazole-5-thioketone compound
A technology of compound and acridine, which is applied in the field of medicine, can solve problems that have not been seen before, and achieve the effects of improving binding ability, increasing conjugation area, and significant in vitro anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation method of 4-(2-methoxyacridinyl)-3-p-nitrophenyl-1,2,4-triazole-5-thione, the specific steps are as follows:
[0030] 1) In a 250ml three-necked flask, add 5.20g (26mmol) of o-bromobenzoic acid (26mmol), p-methoxyaniline (34mmol), 7.5g (36.2mmol) of potassium carbonate and 0.3g (4.7mmol) of copper powder, and then add 30ml Isoamyl alcohol was used as a solvent, refluxed and stirred at 140°C for 2 hours; after the reaction, the solvent was evaporated under reduced pressure, and the obtained residue was added with 600ml of water, reacted at 80°C for 20min, filtered while hot, washed the filter cake, combined the water layer, and used the water layer Concentrated hydrochloric acid was acidified to PH2, and a large amount of light green precipitate was precipitated, which was filtered by suction, and the obtained solid was recrystallized with chloroform to obtain compound 1 with a yield of 79%;
[0031] 2) In a 100ml round bottom flask, add 14.38g (18mmol) o...
Embodiment 2
[0039] Repeat Example 1, the difference is:
[0040] In step 1), change the consumption of potassium carbonate to 5.39g (26mmol), copper powder 0.17g (2.6mmol), replace isoamyl alcohol with n-amyl alcohol, change the temperature during reflux to 150°C, and combine the water The layer was acidified to pH=1 with concentrated hydrochloric acid;
[0041] In step 3), the consumption of sodium thiocyanate is changed to 1.62g (20mmoL), the temperature during reaction is changed to 70°C, and the organic solvent acetone is replaced with methanol;
[0042] In step 4), methanol is used to replace the organic solvent absolute ethanol, and the temperature during reflux is changed to 70°C;
[0043] In step 5), the amount of sodium carbonate was changed to 3g, and it was added to the reaction system in the form of 20w / w% sodium carbonate aqueous solution.
[0044] The separated pale yellow solid was analyzed by mass spectrometry and carbon spectrometry, and it was determined to be 4-(2-met...
Embodiment 3
[0046] Repeat Example 1, the difference is:
[0047] In step 1), the temperature at reflux was changed to 160° C., and the combined aqueous layer was acidified to pH=3 with concentrated hydrochloric acid;
[0048] In step 3), replace organic solvent acetone wherein with acetonitrile;
[0049] In step 4), change the consumption of p-nitrobenzohydrazide to 0.72g (4mmol), replace the organic solvent wherein with the composition of methanol and ethanol (the volume ratio of methanol and ethanol is 5:1) ethanol.
[0050] The separated pale yellow solid was analyzed by mass spectrometry and carbon spectrometry, and it was determined to be 4-(2-methoxyacridinyl)-3-p-nitrophenyl-1,2,4-triazole-5-thione .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com