Carbazole reaction type fluorine ion fluorescent probe based on aryl alkene nitrile as well as preparation method and application of carbazole reaction type fluorine ion fluorescent probe
A technology of fluorescent probes and fluoride ions, applied in the field of detection, can solve the problems of difficulty in fluoride ion detection, low sensitivity, limitations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
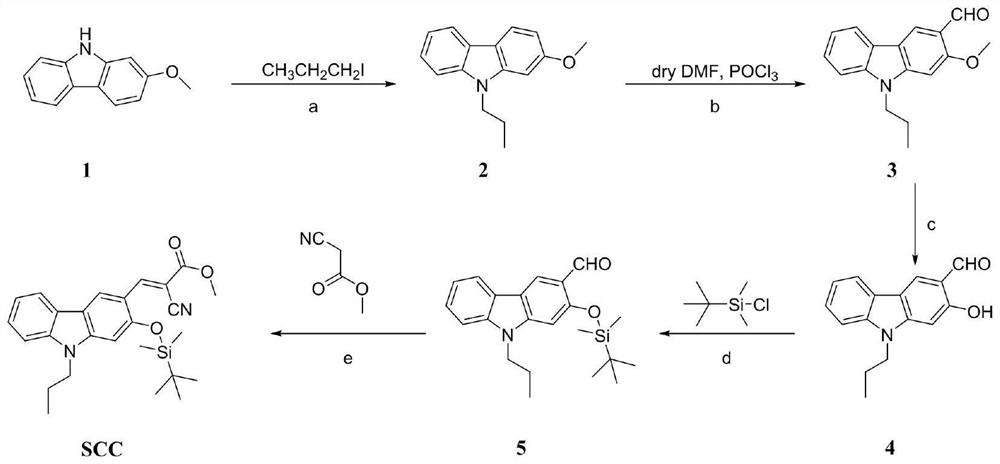
[0035] A method for preparing a carbazole-type reactive fluoride ion fluorescent probe based on acrylonitrile, comprising the following steps:
[0036] (a) Preparation of 2-methoxy-9-propyl-9H-carbazole
[0037] In a 100 mL round bottom flask, 2-methoxy-9H-carbazole (5.91 g, 30 mmol) and sodium hydroxide (4.2 g, 105 mmol) were added and dissolved in DMSO (60 mL), and iodopropane ( 10.2 g, 60 mmol). After all the raw materials were added, a condenser tube and an Ar balloon were placed above the round-bottomed flask, and the temperature was raised to 65 °C for 12 h after vacuuming for three times. After the reaction was completed, it was cooled to room temperature, diluted with 60 mL of water, extracted with dichloromethane (60 mL×3), the organic phases were combined and washed with saturated NaCl solution (100 mL×2), then anhydrous sodium sulfate was added and left to stand for 15 min, and the mixture was rotated under reduced pressure. The crude product was obtained by dryin...
Embodiment 2
[0047] A method for preparing a carbazole-based reactive fluoride ion fluorescent probe based on acrylonitrile, comprising the following steps:
[0048] (a) Preparation of 2-methoxy-9-propyl-9H-carbazole
[0049] In a 100 mL round bottom flask, 2-methoxy-9H-carbazole (4.93 g, 25 mmol) and sodium hydroxide (4.0 g, 100 mmol) were added and dissolved in DMSO (50 mL), and iodopropane ( 10.6 g, 62.5 mmol). After all the raw materials were added, a condenser tube and an Ar balloon were placed above the round-bottomed flask, and the temperature was raised to 65 °C for 14 h after vacuuming for three times. After the reaction was completed, it was cooled to room temperature, diluted with 50 mL of water, extracted with dichloromethane (50 mL × 3), the organic phases were combined and washed with saturated NaCl solution (100 mL × 2), then anhydrous sodium sulfate was added and allowed to stand for 15 min, and then rotated under reduced pressure. The crude product was obtained by drying...
Embodiment 3
[0059] A method for preparing a carbazole-type reactive fluoride ion fluorescent probe based on acrylonitrile, comprising the following steps:
[0060] (a) Preparation of 2-methoxy-9-propyl-9H-carbazole
[0061] In a 100 mL round bottom flask, 2-methoxy-9H-carbazole (5.91 g, 30 mmol) and potassium hydroxide (8.4 g, 150 mmol) were added and dissolved in DMSO (60 mL), and iodopropane ( 15.3 g, 90 mmol). After all the raw materials were added, a condenser tube and an Ar balloon were placed above the round-bottomed flask, and the temperature was raised to 60 °C for 16 h after vacuuming for three times. After the reaction was completed, it was cooled to room temperature, diluted with 60 mL of water, extracted with dichloromethane (60 mL × 3), the organic phases were combined and washed with saturated NaCl solution (100 mL × 2), anhydrous sodium sulfate was added and allowed to stand for 15 min, and the mixture was rotated under reduced pressure. The crude product was obtained by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com