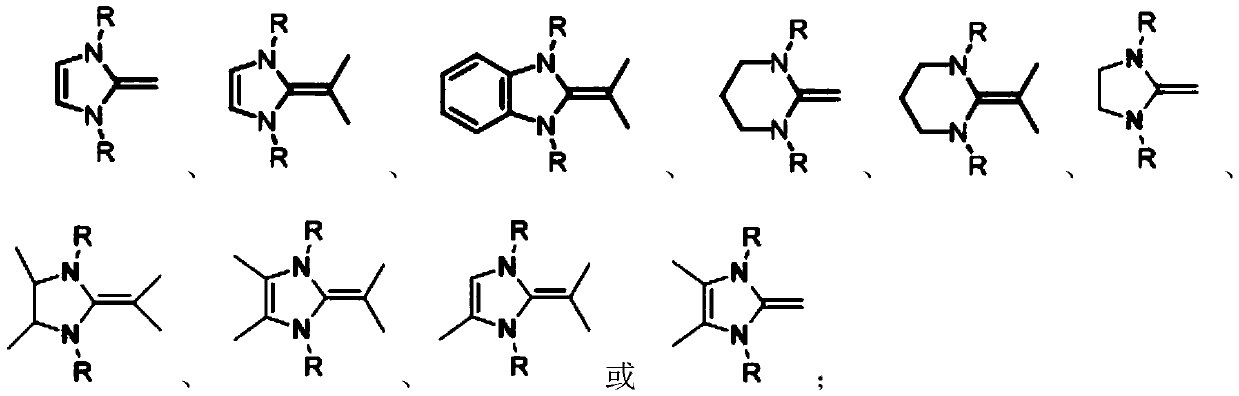
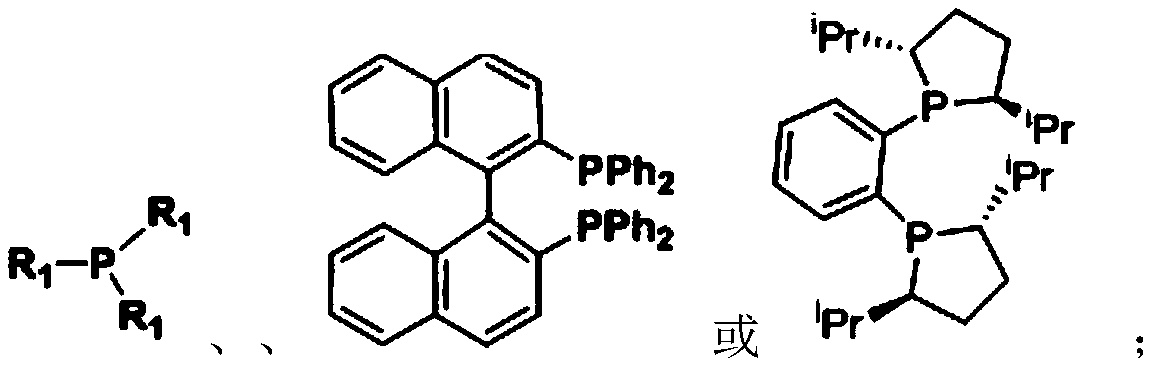
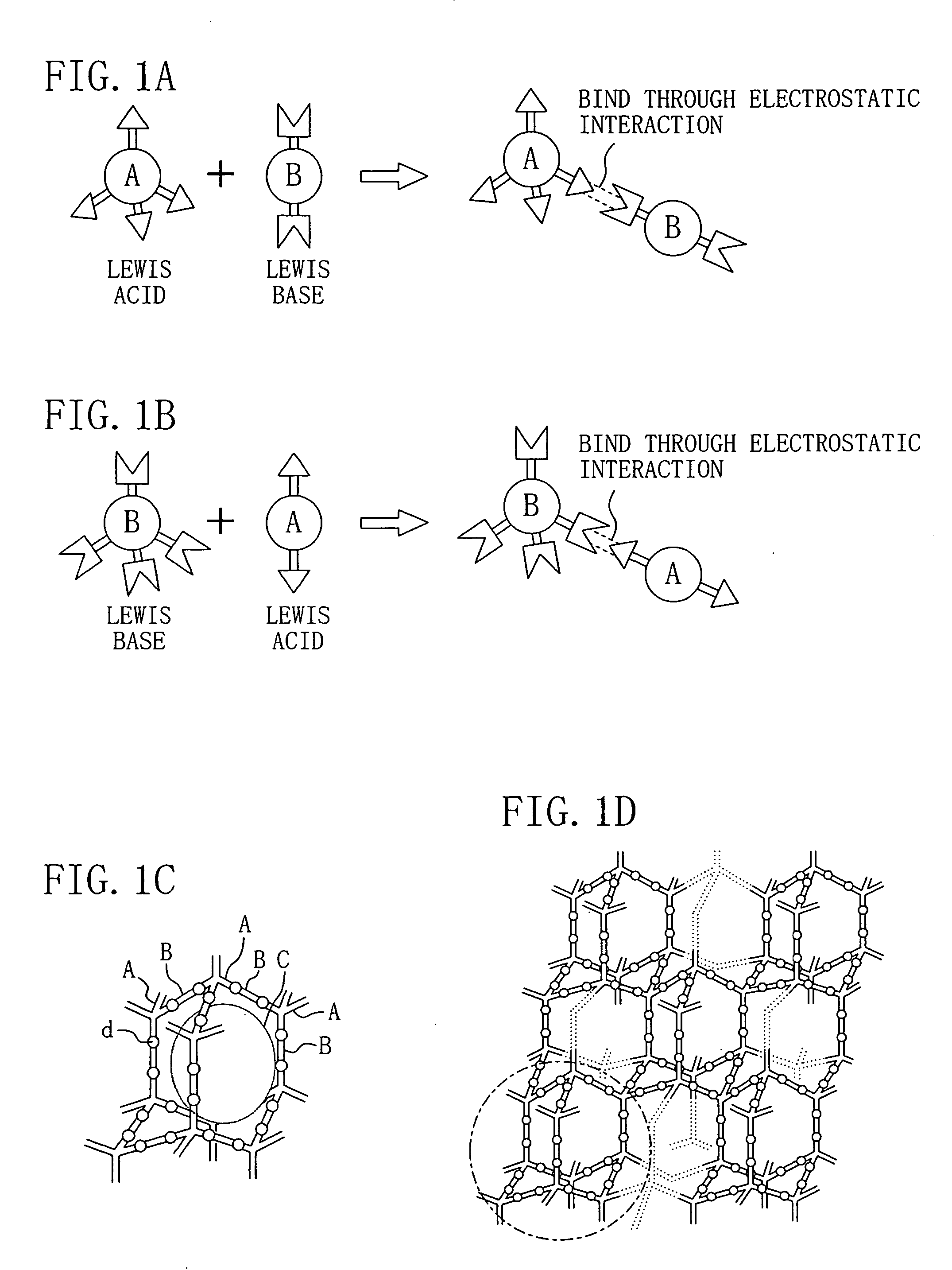
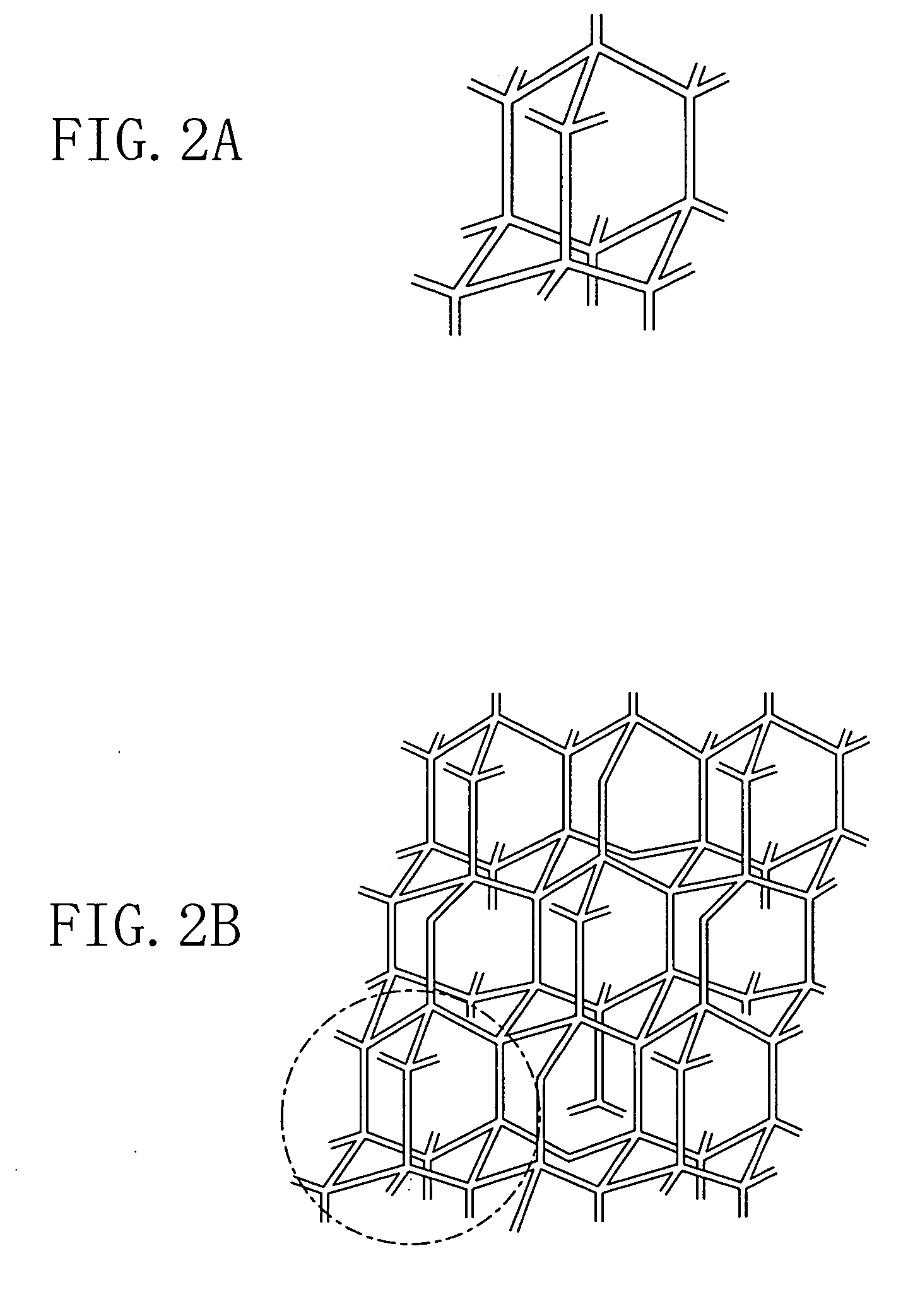
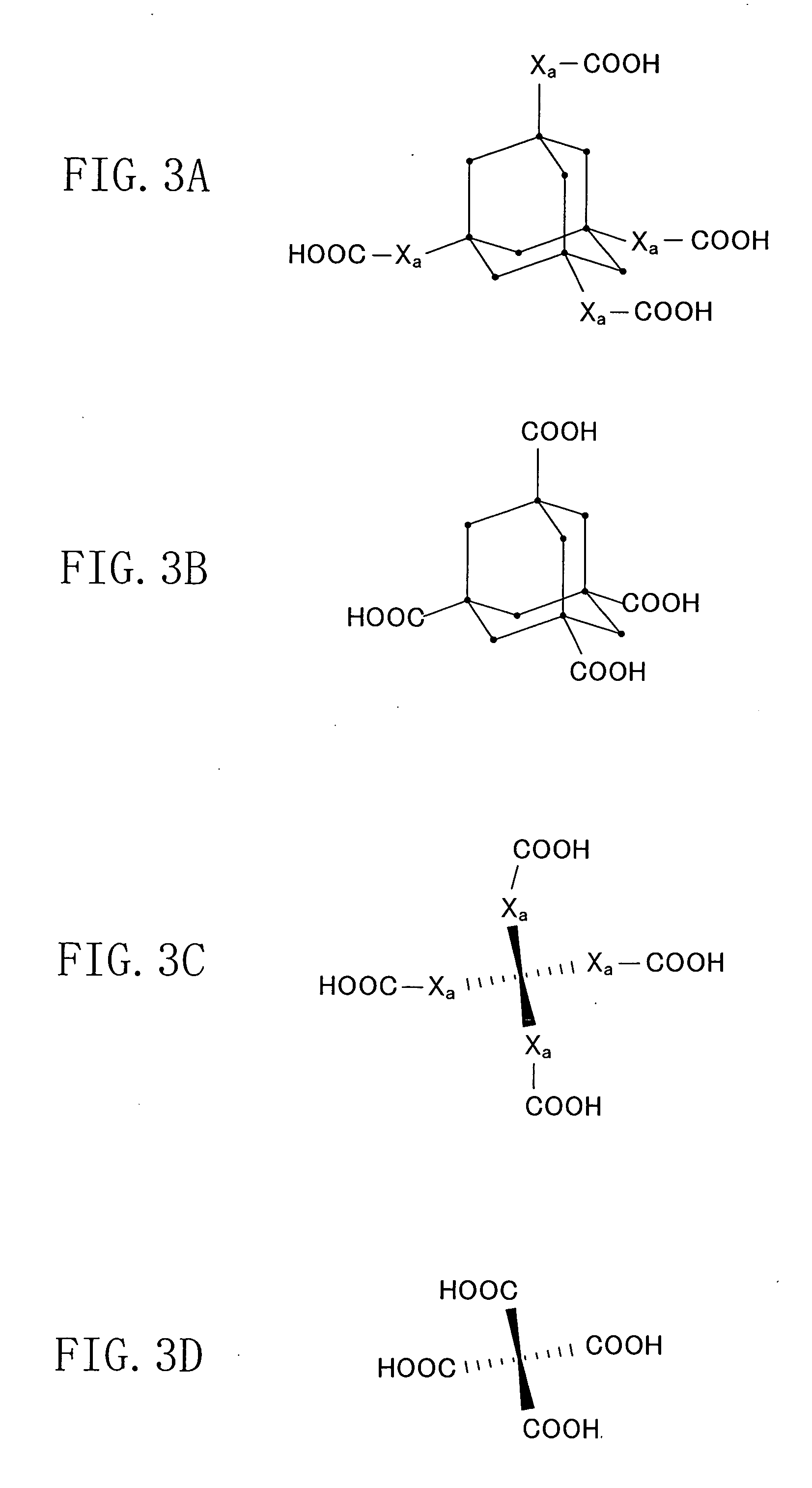
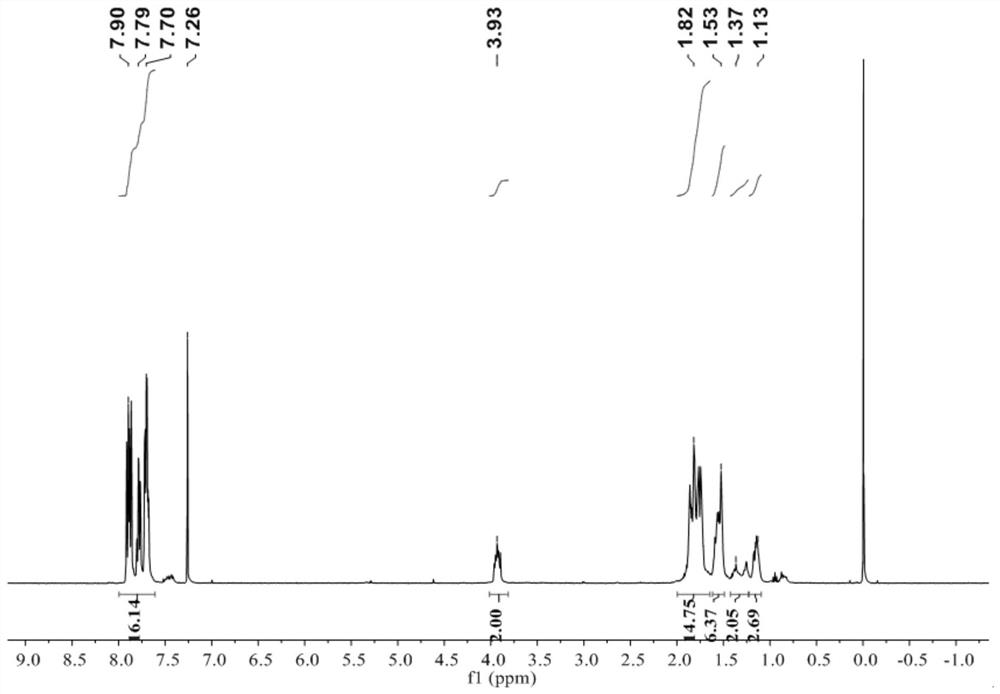
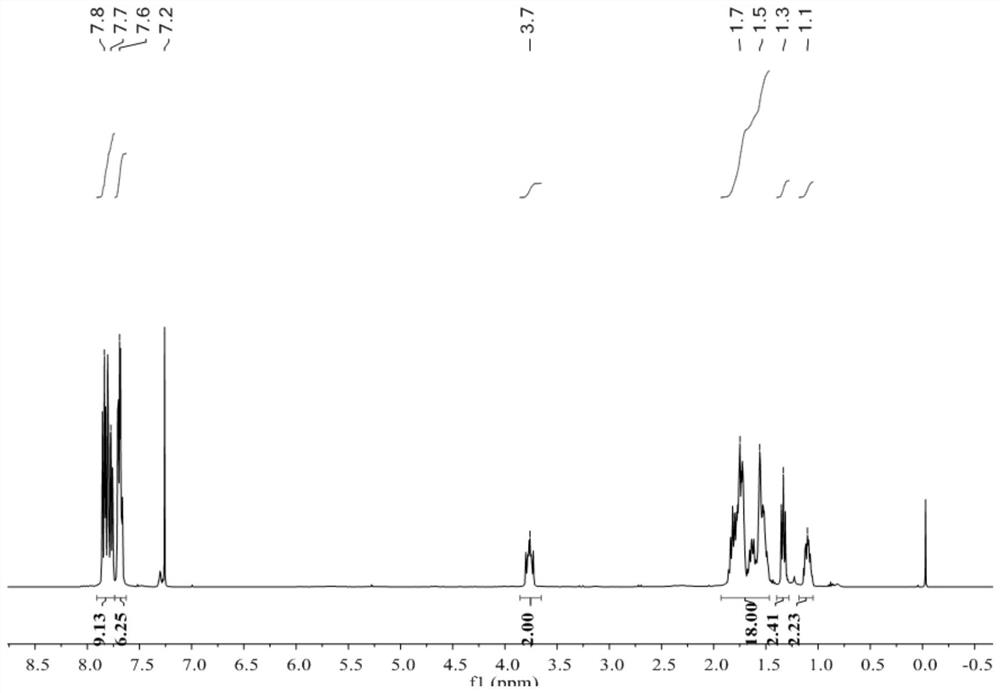
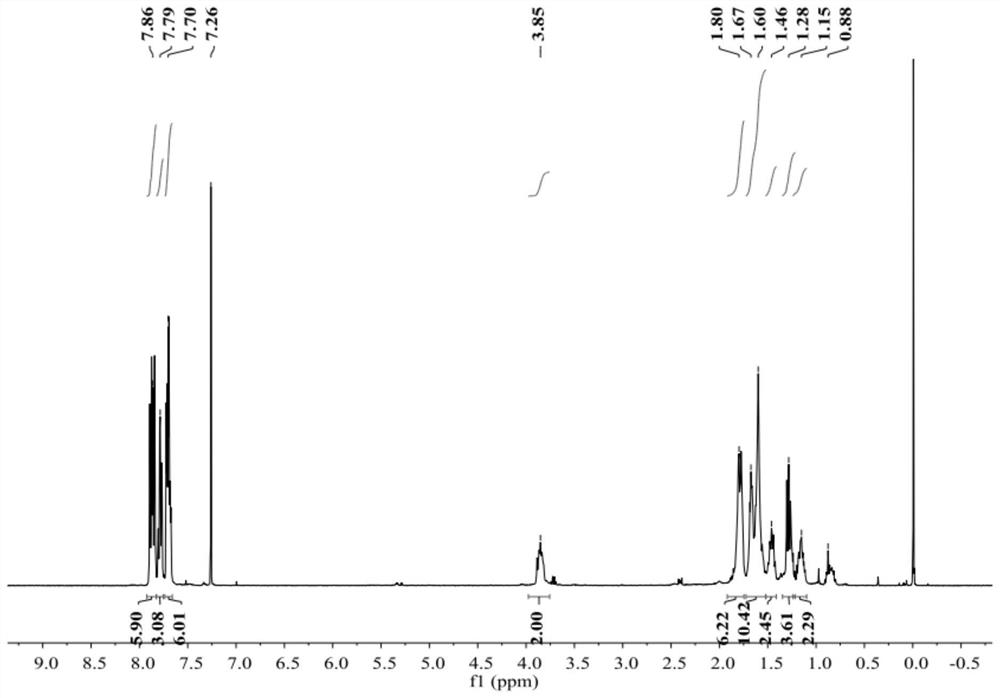
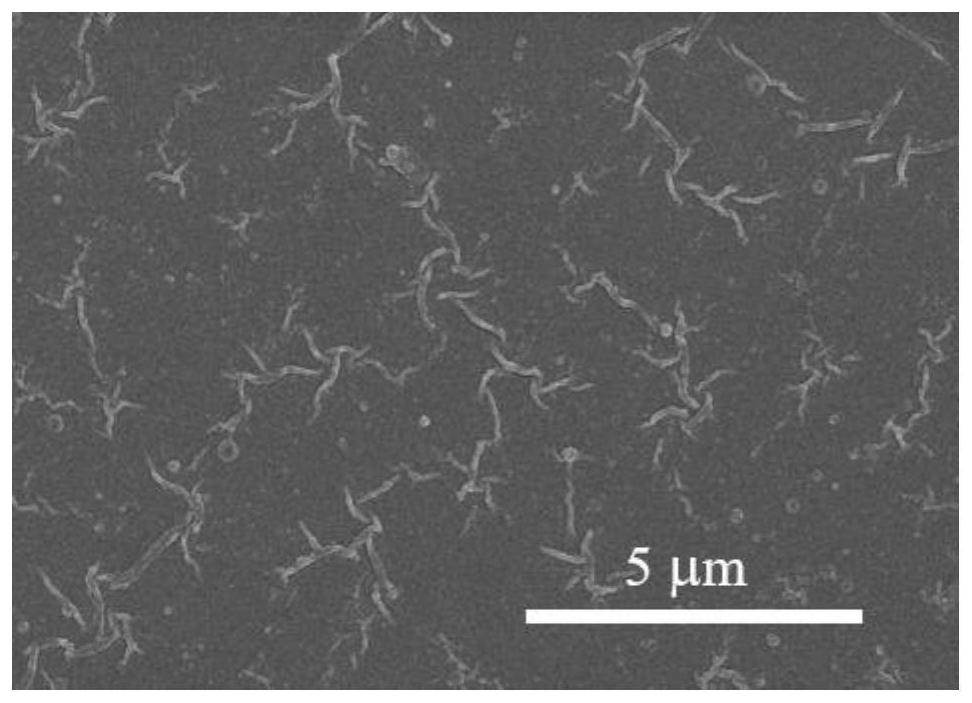
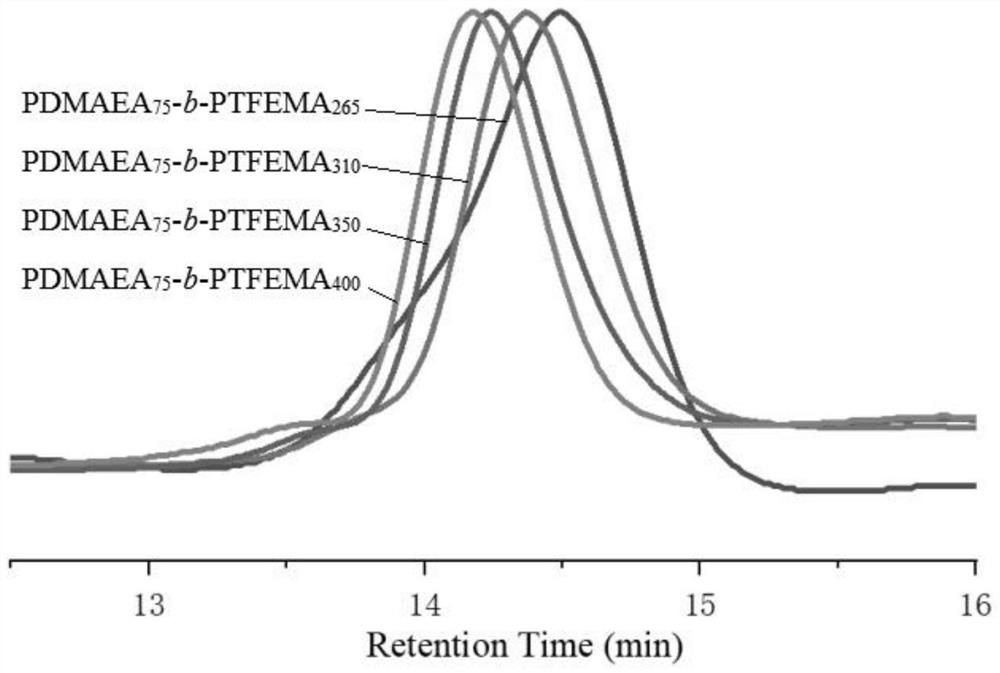
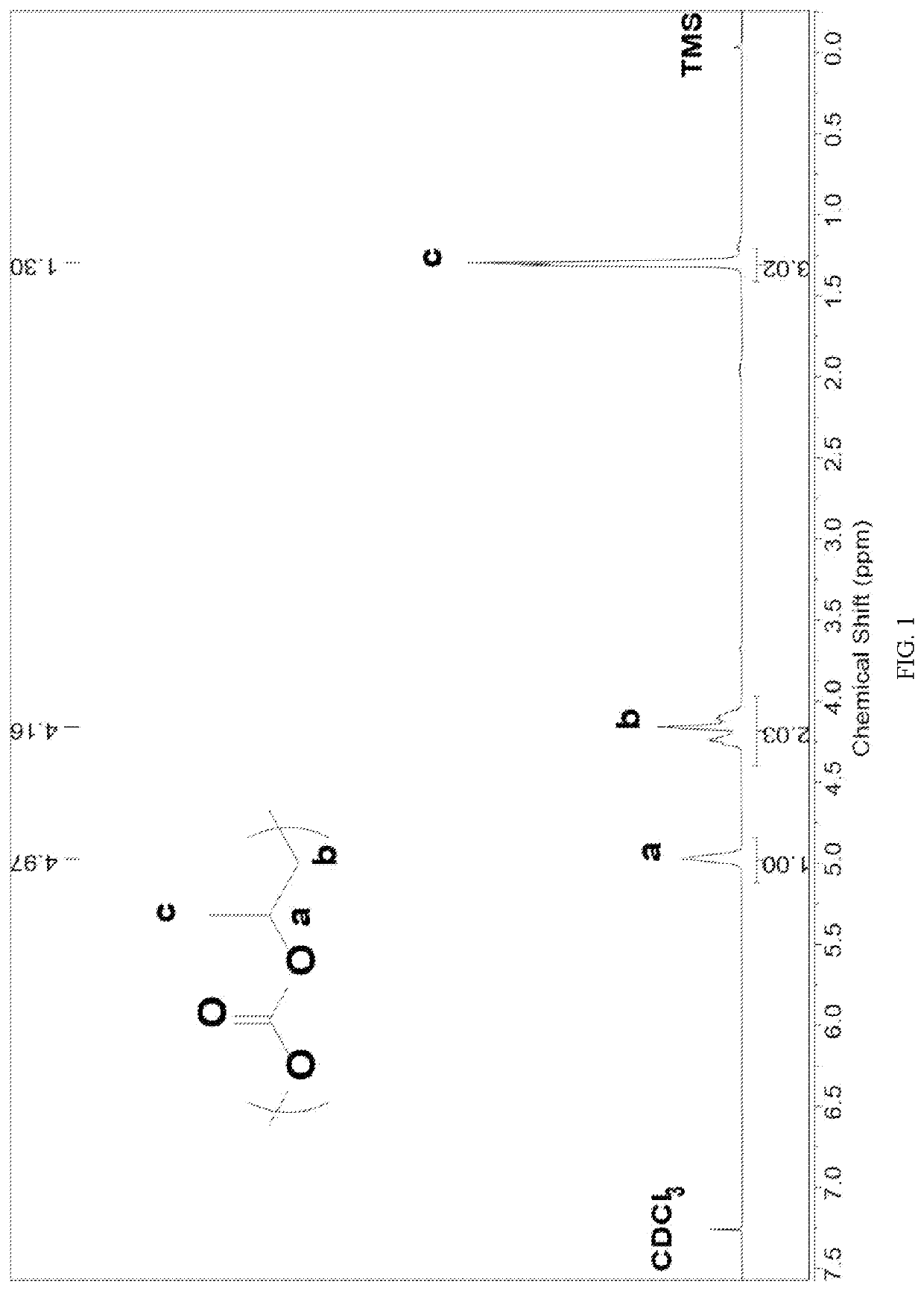
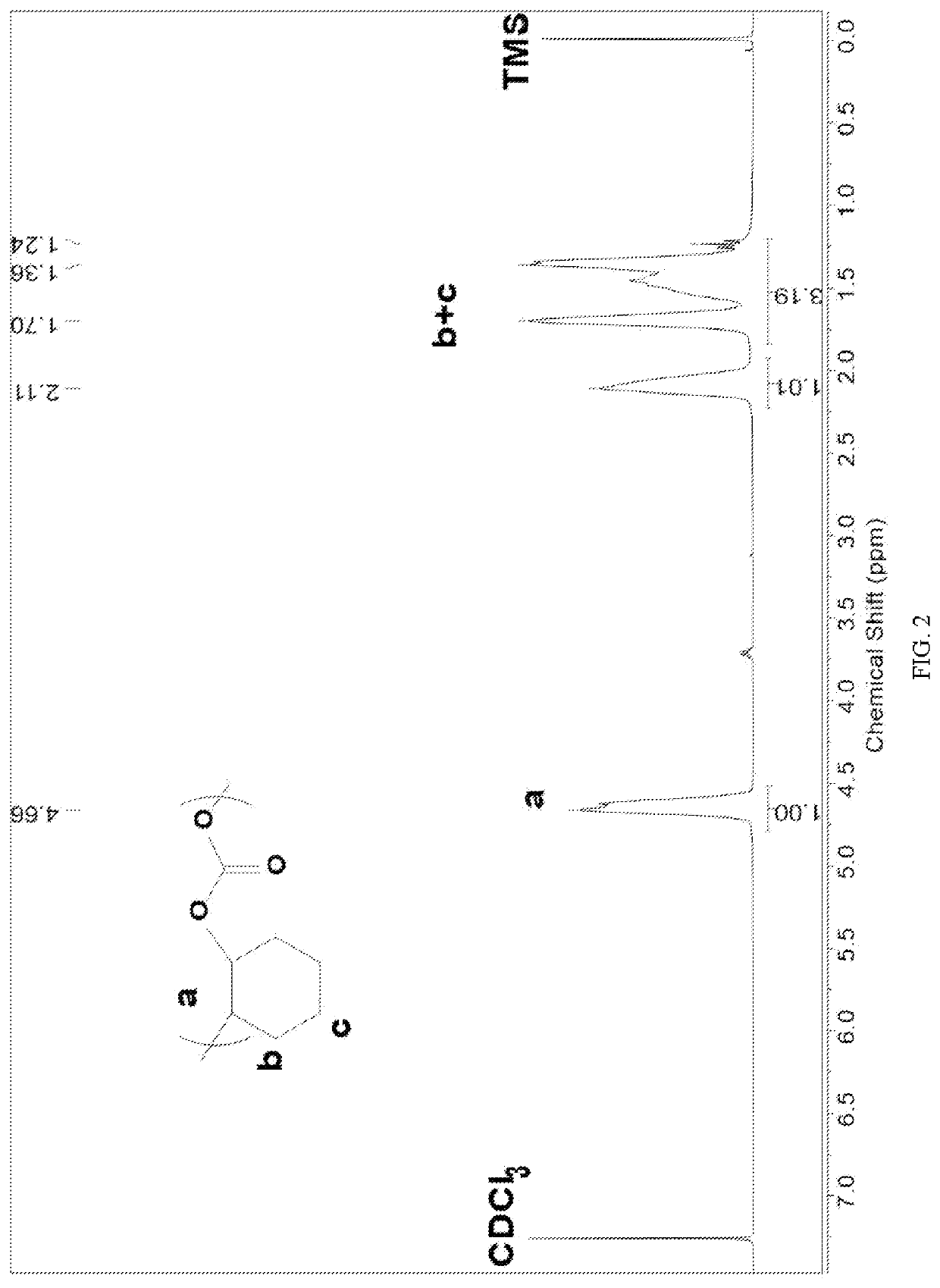
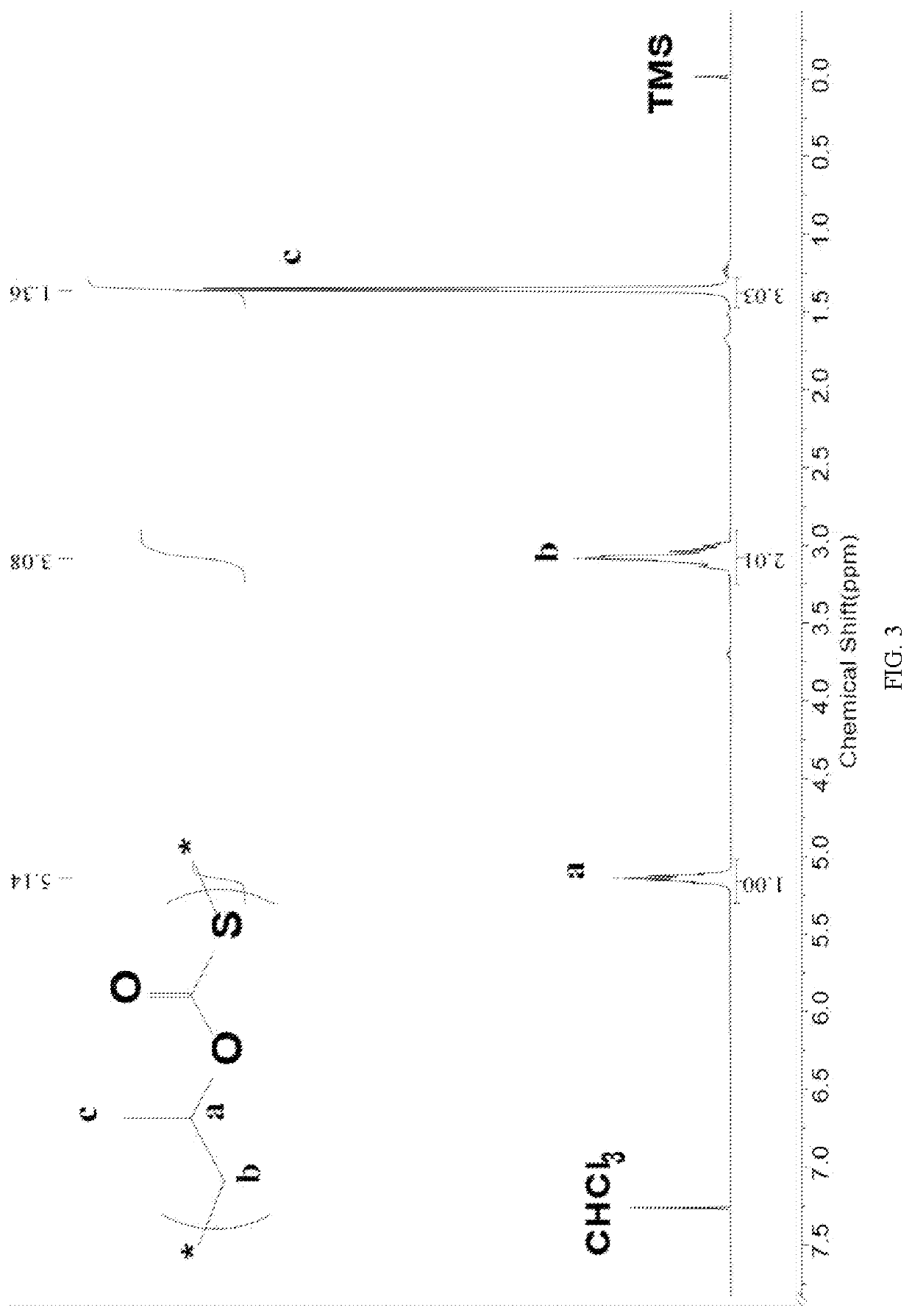
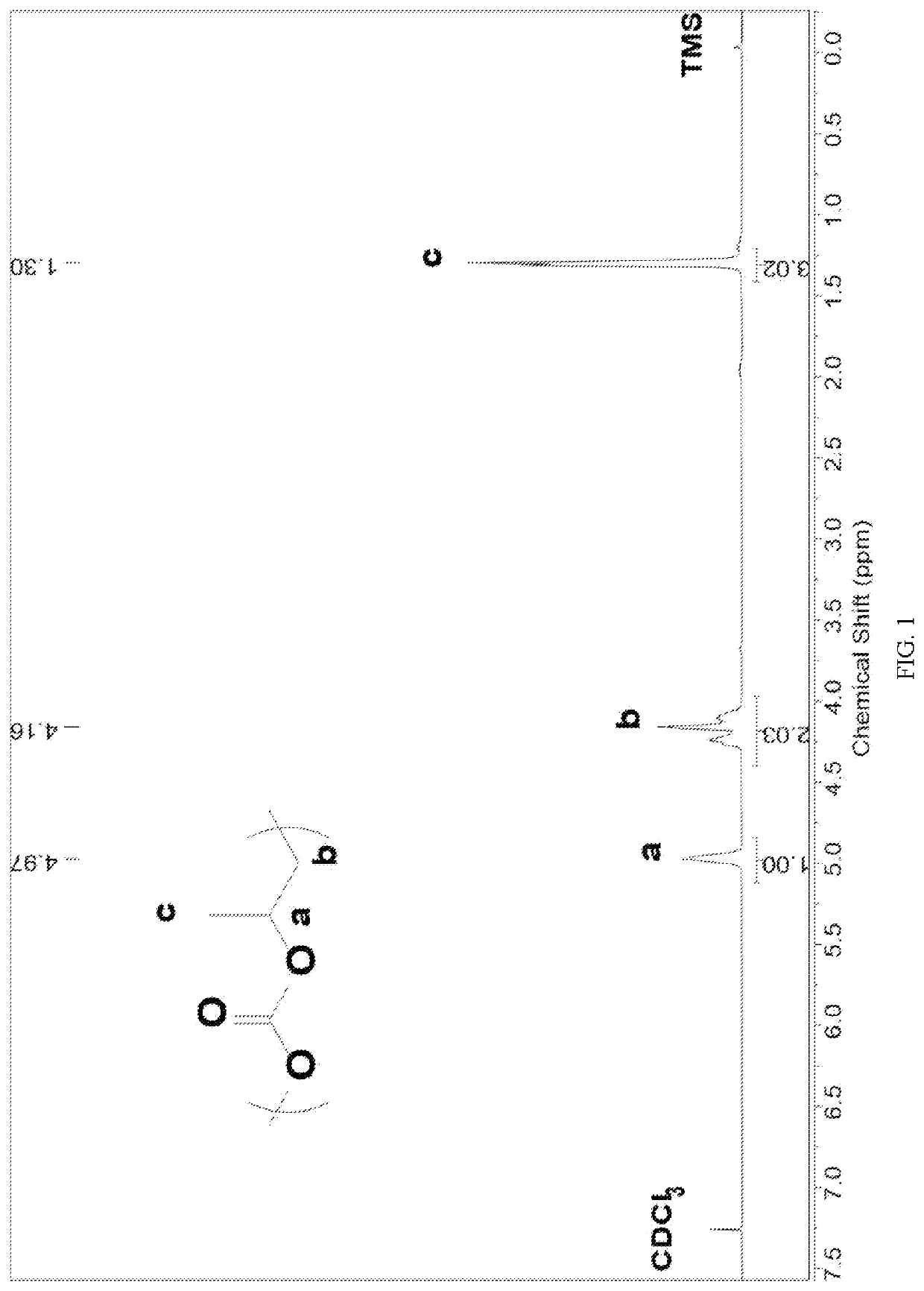
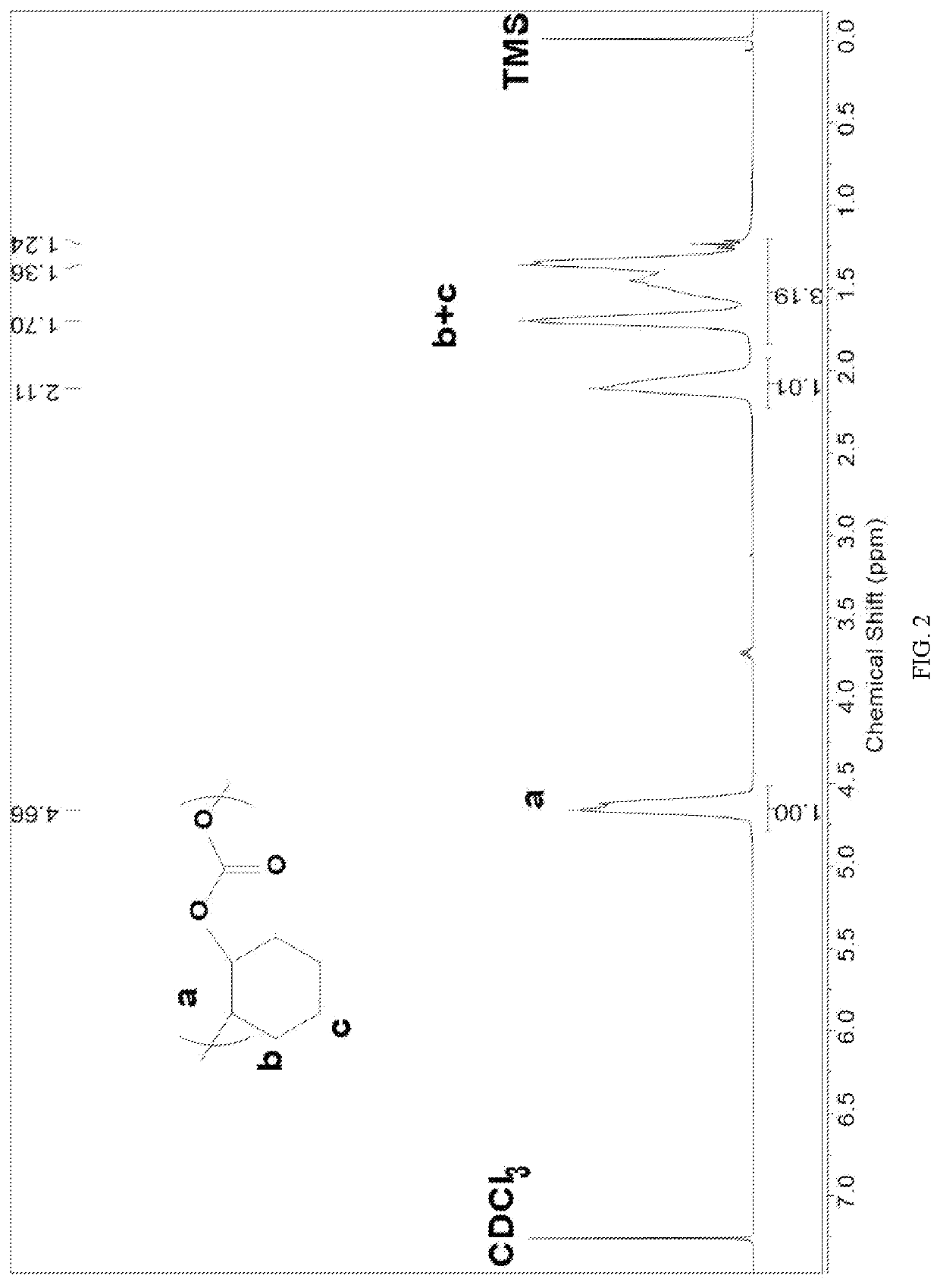
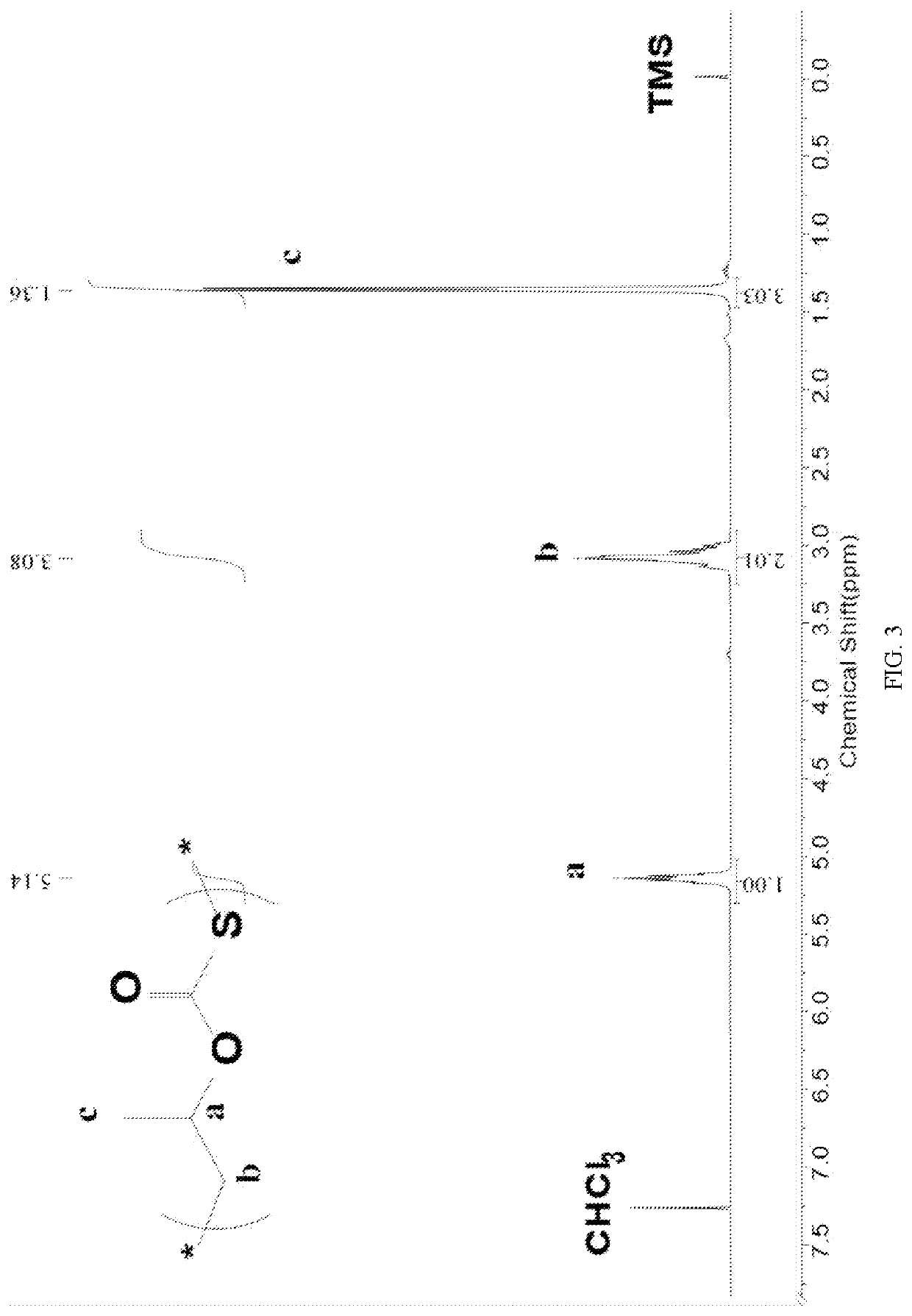
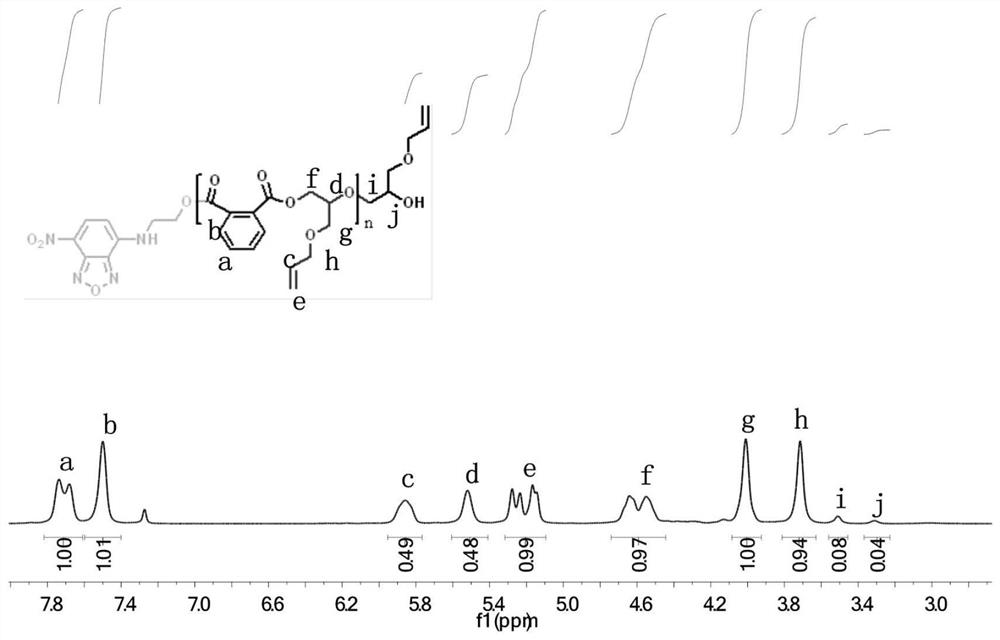
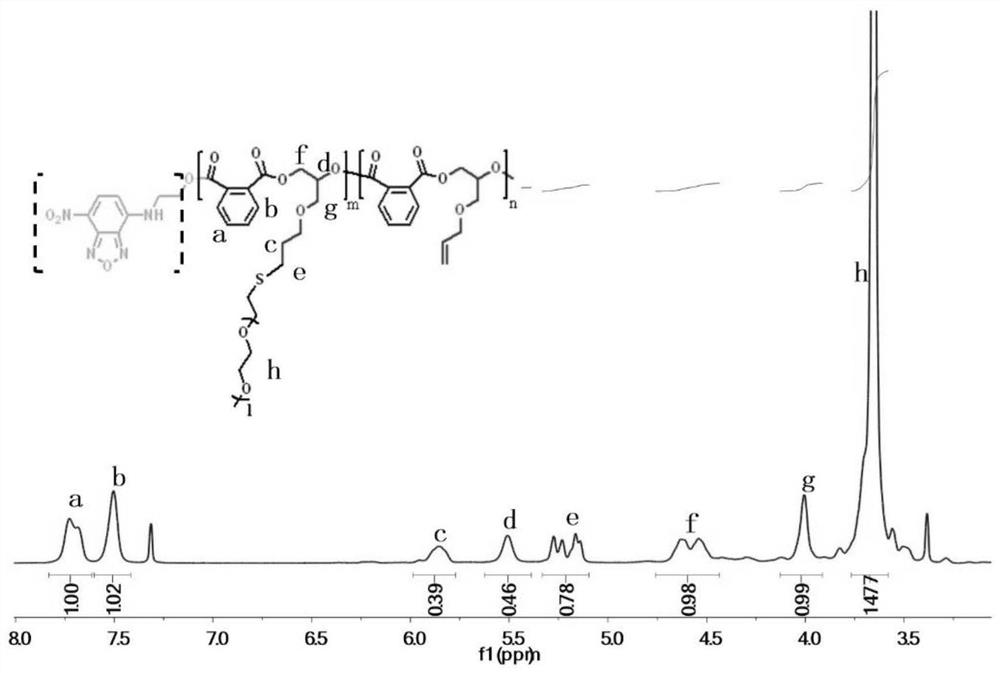
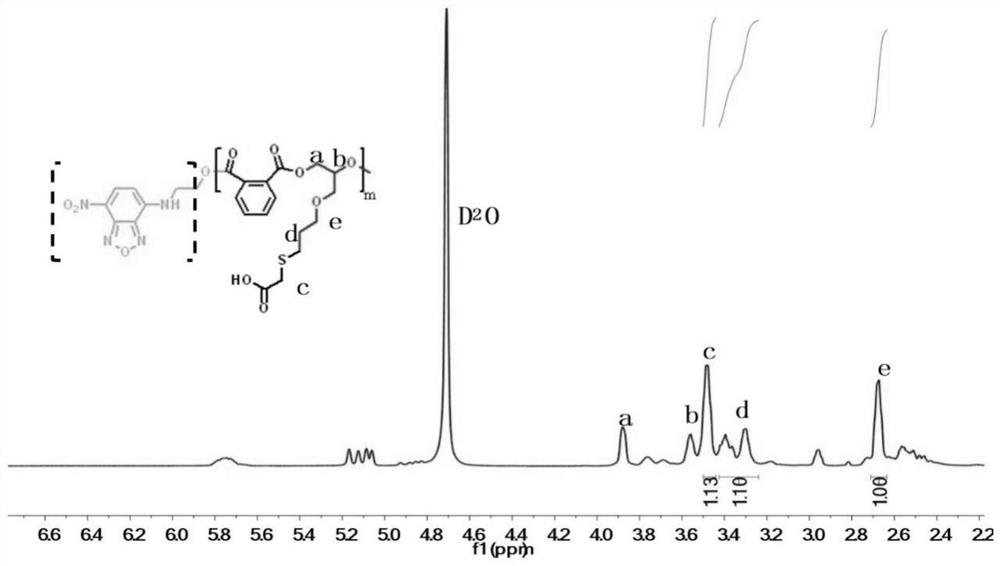
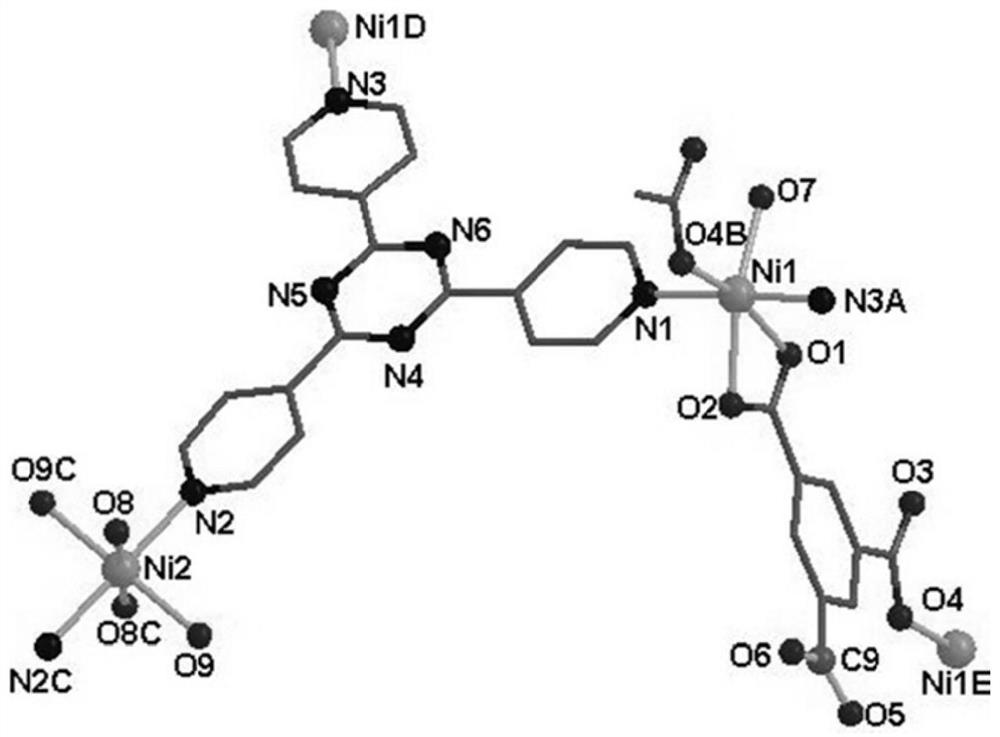
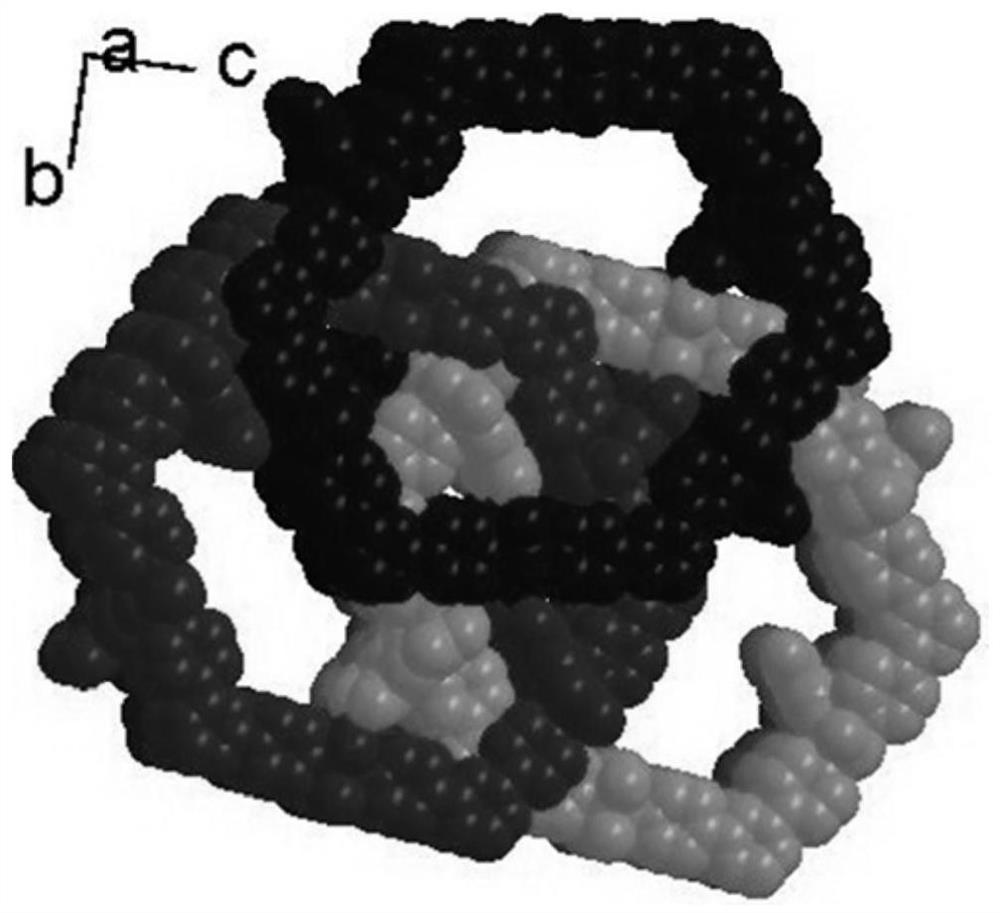
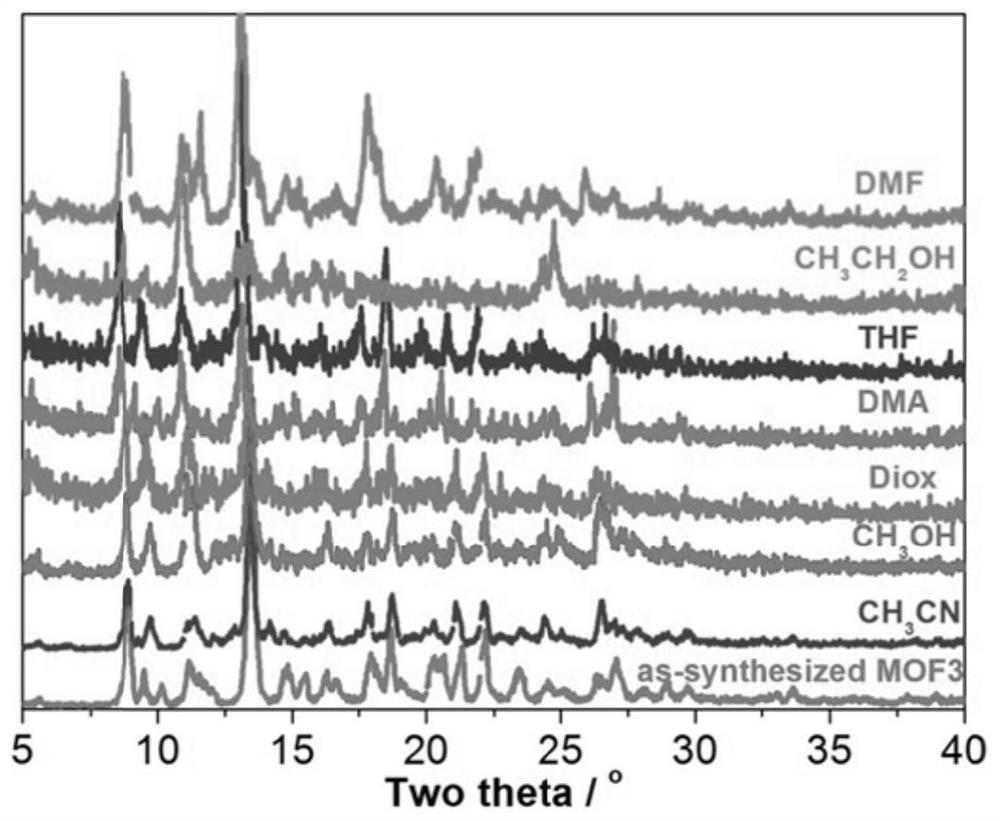
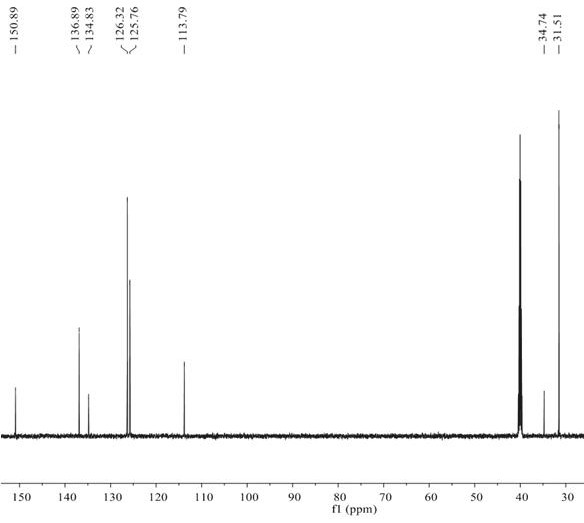
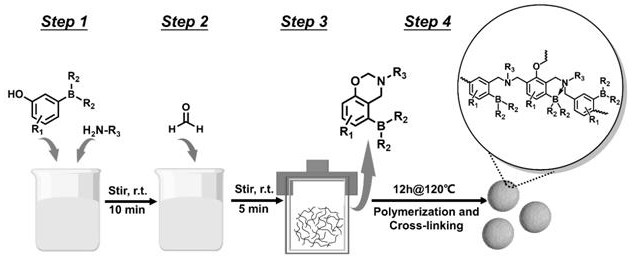
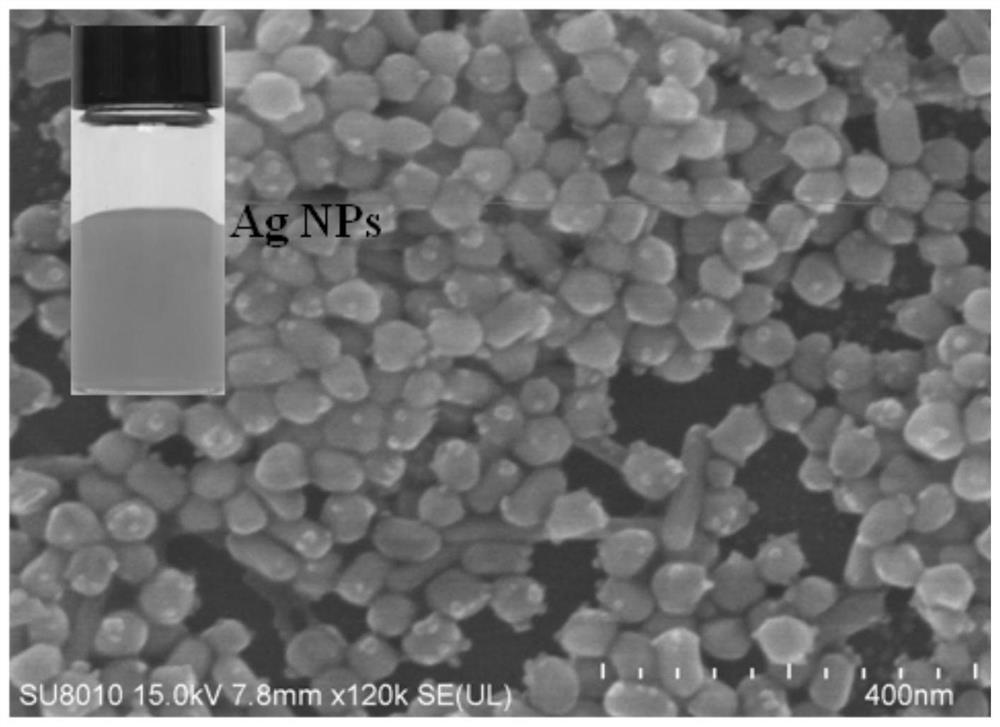
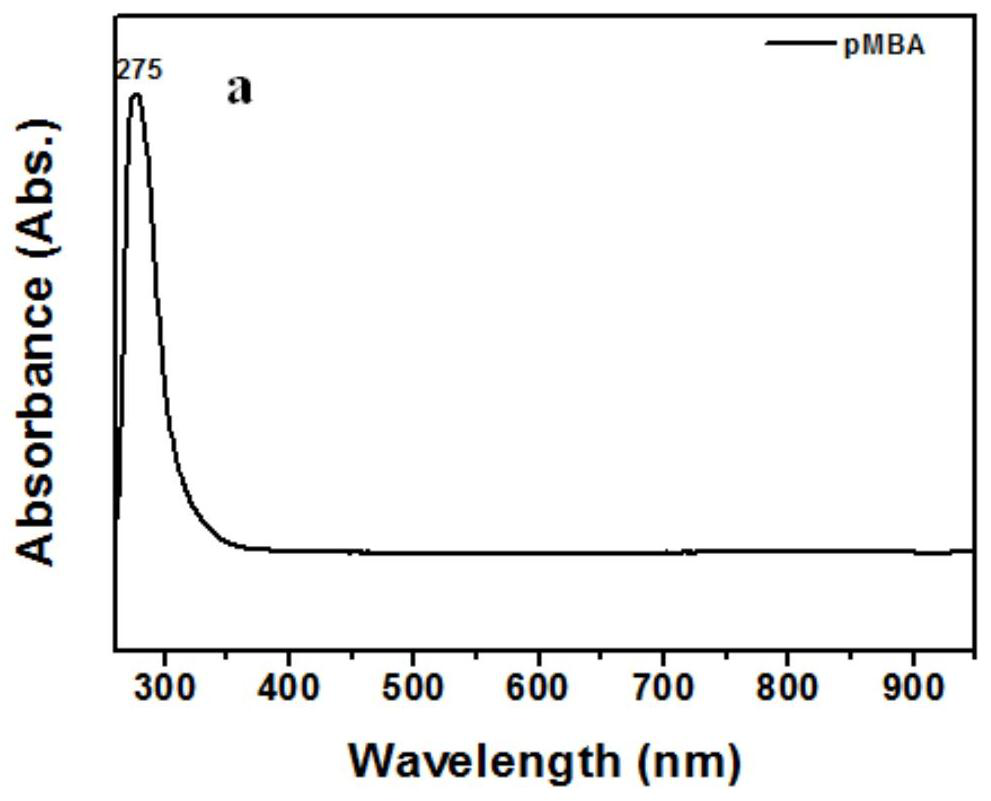
Patents
Literature
61 results about "Lewis acids and bases" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A Lewis acid is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH₃ is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane (Me₃B) is a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH₃ and Me₃B, the lone pair from NH₃ will form a dative bond with the empty orbital of Me₃B to form an adduct NH₃•BMe₃. The terminology refers to the contributions of Gilbert N. Lewis.
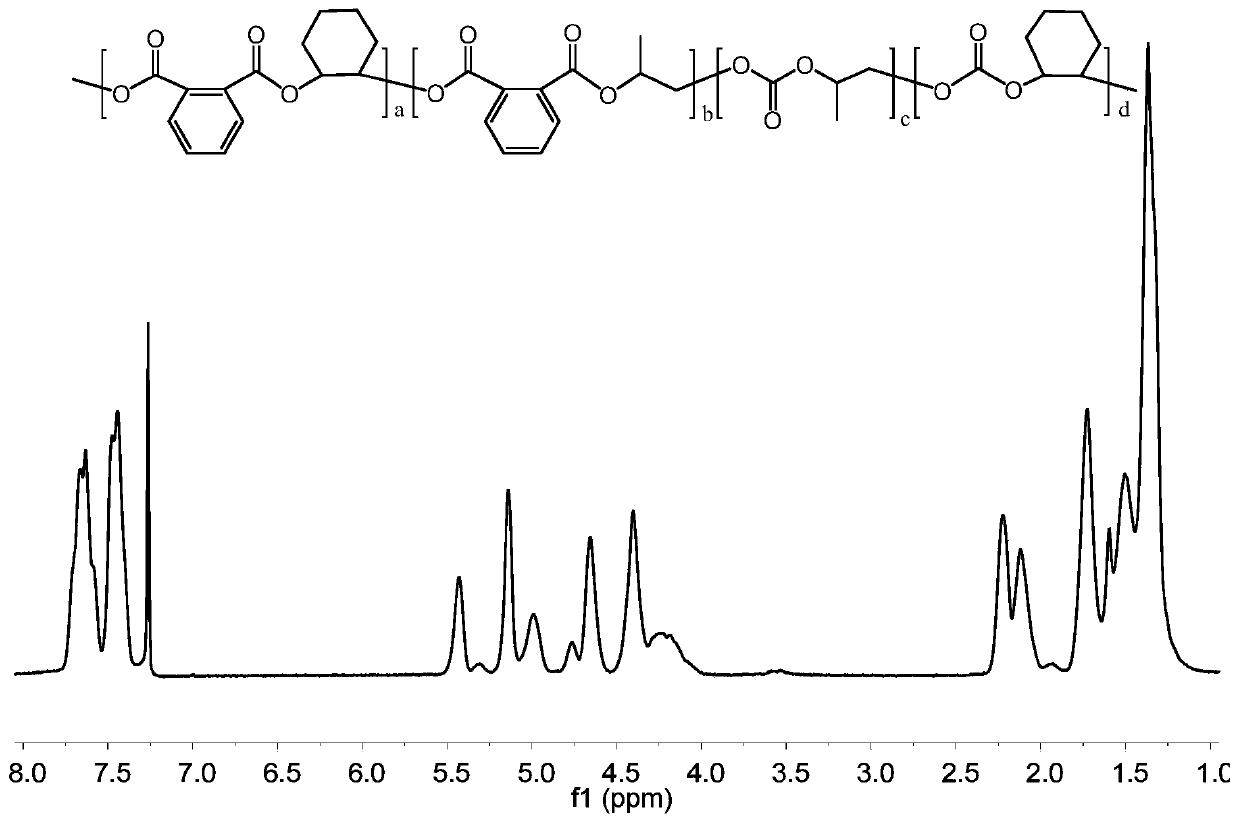
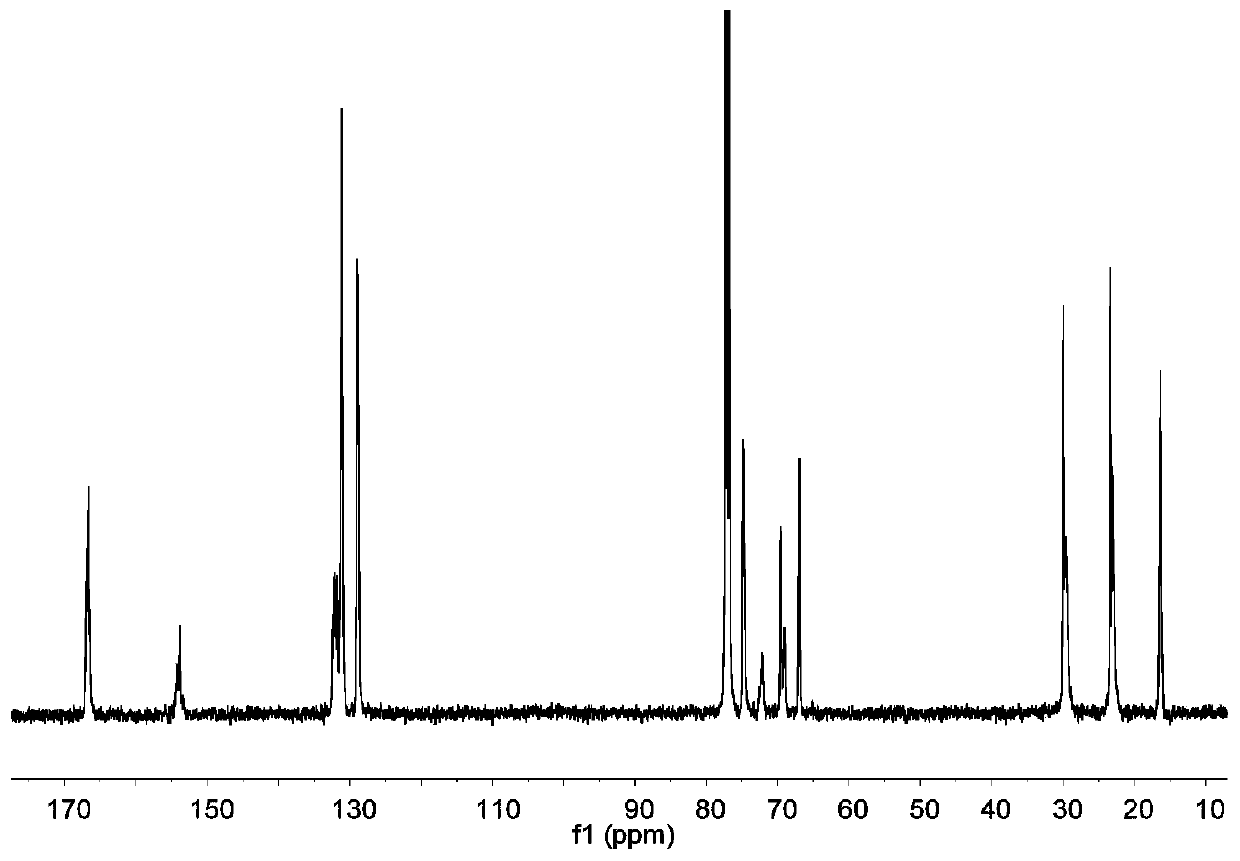
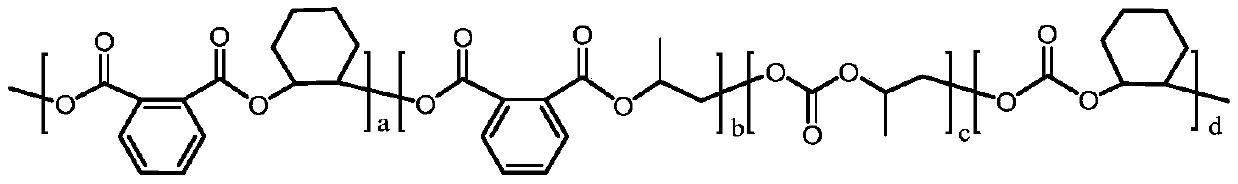
Preparation method of carbon-dioxide-based polyester-polycarbonate quadriblock copolymer
PendingCN111333825ASolve residual problemsHigh glass transition temperaturePolymer sciencePtru catalyst
The invention relates to the technical field of polymer material synthesis, particularly to a preparation method of a carbon-dioxide-based biodegradable polyester-polycarbonate quadriblock copolymer.The block copolymer comprises a diblock copolymer (A-B type); the chain segment A is polycarbonate obtained by ring-opening polymerization of epoxypropane, cyclohexene oxide and CO2, and the chain segment B is aromatic polyester obtained by ring-opening polymerization of epoxypropane, cyclohexene oxide and phthalic anhydride. Commercialized Lewis acid-base pairs are used as catalysts; phthalic anhydride and cyclohexene oxide are successfully introduced into a main chain of polymethyl ethylene carbonate (PPC) through a one-pot one-step method, so that the glass-transition temperature, the thermal stability and the tensile strength of the PPC are greatly improved; and a problem of metal catalyst residues in the prior art is solved, and the application range of the PPC material is expanded.
Owner:SUN YAT SEN UNIV
Special catalyst for preparing p-chlorobenzonitrile by ammonoxidation process, preparation method and use
ActiveCN109847772AImprove thermal stabilityHigh mechanical strengthPhysical/chemical process catalystsPreparation by hydrocarbon ammoxidationAdditive ingredientFixed bed
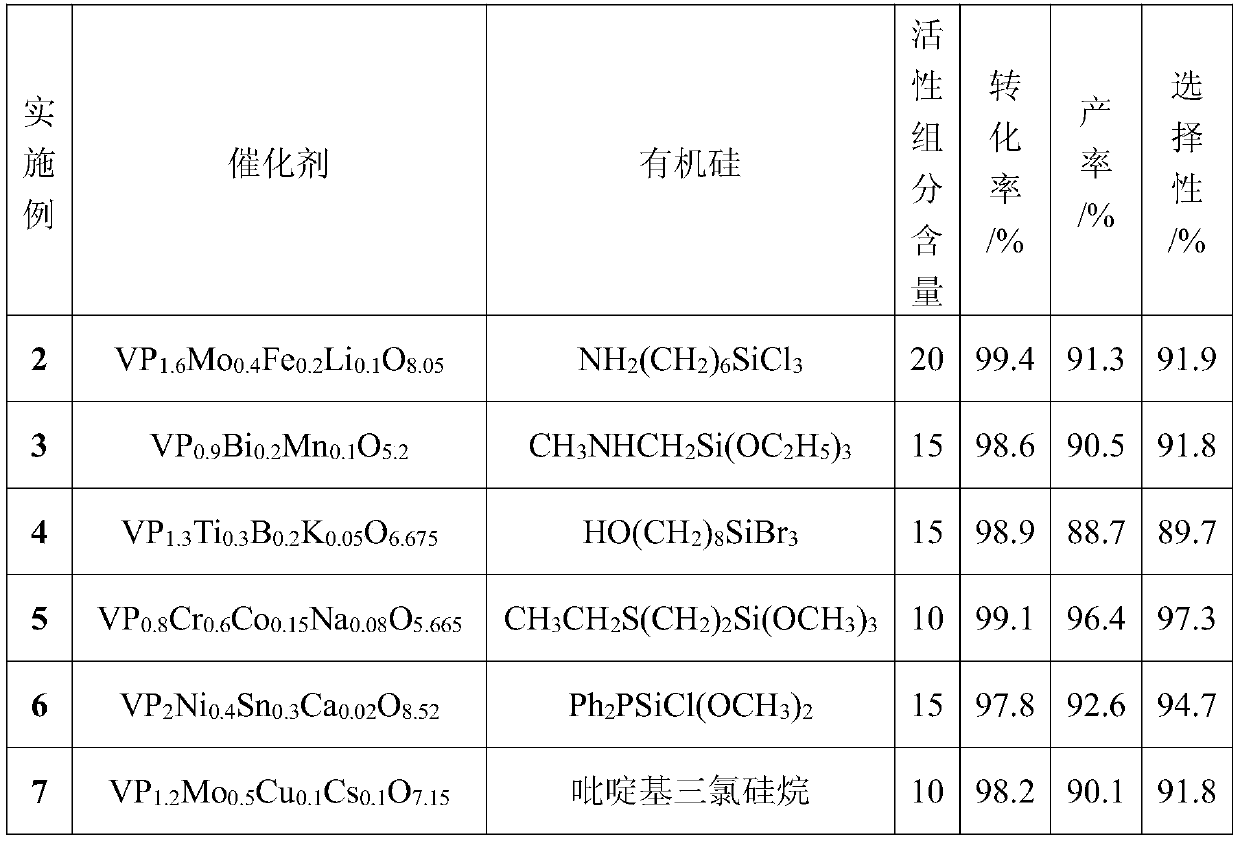
The invention discloses a special catalyst for preparing p-chlorobenzonitrile by an ammonoxidation process. Organosilicone modified silica gel serves as a carrier of the special catalyst, a main catalyst comprises vanadium and phosphorus, a cocatalyst comprises at least one of an ingredient G, an ingredient D and an ingredient E, and an active ingredient of the special catalyst is represented as VPbGcDdEeOx; and the G is molybdenum, chromium, titanium, nickel or bismuth, the D is boron, manganese, ferrum, cobalt, copper, zinc or tin, and the E is potassium, lithium, sodium, cesium, magnesium or calcium. The invention further discloses a preparation method for the catalyst and use of the catalyst. According to the special catalyst, the preparation method and the use, electron donating groups on organosilicone and inorganic elements are subjected to an Lewis acid alkali reaction, and thus, actions of inorganic oxides and the carrier are strengthened; and meanwhile, the inorganic oxides can be more uniformly dispersed, catalyst ingredients are low in loss, the catalytic activity is high, the selectivity is good, and the service life of a commercial catalyst is prolonged to 2 years ormore from 1 year. The catalyst is simple in preparation method, low in cost and good in thermal stability and mechanical strength and can be applied to fixed-bed and fluidized-bed reactors.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
MgAl LDO/nitrogen vacancy carbon nitride-based photocatalyst as well as preparation method and application thereof
ActiveCN112774714AImprove cycle stabilitySimple processPhysical/chemical process catalystsCarbon monoxidePhysical chemistryCarbon nitride
The invention belongs to the field of nano materials, and relates to an MgAl LDO / nitrogen vacancy carbon nitride-based photocatalyst as well as a preparation method and application thereof. According to the preparation method, a carbon nitride (g-C3N4) nanosheet rich in nitrogen vacancies is prepared, magnesium-aluminum bimetallic layered hydroxide (MgAl LDH) is deposited on the surface of the carbon nitride (g-C3N4) nanosheet in situ, and the magnesium-aluminum bimetallic layered oxide (MgAl LDH) / nitrogen vacancy carbon nitride composite photocatalytic CO2 reduction material which is low in cost, easy to obtain, stable in structure and high in catalytic activity is prepared through continuous calcination. The surface of MgAlLDO has abundant Lewis base / acid sites, O<2->, Mg<2+>-O<2-> and OH groups are used as Lewis base sites to improve the adsorption activation capacity of CO2, Al<3+> and Al<3+>-O<2-> are used as Lewis acid sites to improve the adsorption / dissociation of H2O, the oxidation semi-reaction of H2O is accelerated, more protons are provided, and the CO2 reduction activity is further improved. Meanwhile, surface nitrogen vacancies are beneficial to separation of photo-induced electrons and holes, and the Lewis acid / alkali and the nitrogen vacancies cooperate to enable the composite photocatalytic material to have excellent CO2 photocatalytic performance.
Owner:JIANGSU UNIV
Supported metallocene catalyst, method of preparing the catalyst and method of preparing polyolefin using the catalyst
ActiveUS20060052238A1High polymerization activityPreventing reactor foulingMolecular sieve catalystsOrganic-compounds/hydrides/coordination-complexes catalystsApparent densityPolymer science
Provided are a supported metallocene catalyst which has excellent supporting efficiency due to an interaction between a cocatalyst supported on a carrier and a metallocene compound substituted with a functional group that can function as a Lewis base, and a method of polymerizing an olefin using the supported metallocene catalyst. In the supported metallocene catalyst, the metallocene catalyst is strongly bound to the carrier due to a Lewis acid-base interaction between the metallocene compound and the cocatalyst, and thus the metallocene catalyst is not separated from the carrier during the polymerization of polyolefin in a slurry or gas phase method. Thus, fouling is prevented and the prepared polymer has a good particle shape and a high apparent density. Thus, the supported metallocene catalyst can be suitably used in a conventional slurry or gas phase polymerization process.
Owner:LG CHEM LTD
Efficient and energy-saving method for trapping carbon by ionic liquid
InactiveCN103752134AImprove capture capacityReduced effectProductsCarbon compoundsTrappingSpatial structure
The invention discloses an efficient and energy-saving method for trapping carbon by ionic liquid, which is applied to chemical trapping of carbon dioxide. A high-capacity, low-energy consumption and recyclable trapping of carbon dioxide is realized by using a synergistic effect of Lewis acids and bases and C-H...O, and thus a potential method is provided for industrial trapping of carbon dioxide. Compared with a conventional method, the method adopted by the invention is very novel, and has the following characteristics and beneficial effects: 1, through designing anion functionalized ionic liquid with different space structures and using the synergistic effect of Lewis acids and bases and C-H...O between carbonyl and carbon dioxide, the trapping amount of the ionic liquid to the carbon dioxide is greatly increased, which is up to 1.4mol / mole ionic liquid; and 2, due to the introduction of an electron-withdrawing function of the carbonyl, the acting enthalpy of the ionic liquid and the carbon dioxide is reduced, and the carbon dioxide is more easily desorbed.
Owner:ZHEJIANG UNIV
Heteroatom doped carbon/CoS2 functional material derived based on metal organic framework and application thereof
PendingCN111313111AImprove performanceImprove reaction kineticsLi-accumulatorsCell component detailsPorous carbonMetal-organic framework
The invention discloses a heteroatom doped carbon / CoS2 functional material derived based on a metal organic framework and application of the heteroatom doped carbon / CoS2 functional material. The heteroatom doped carbon / CoS2 functional material is a porous CoS2 / C functional material obtained by performing high-temperature carbonization and gas-phase vulcanization treatment on a designed and synthesized metal organic framework composite structure. The heteroatom doped carbon / CoS2 functional material disclosed by the invention has an excellent chemical adsorption effect on polysulfide, i.e., a Keesom force effect of heteroatom doped carbon and a Lewis acid-base effect of CoS2. Besides, the porous carbon structure also has physical barrier and adsorption effects, and the conductive carbon canalso promote the reaction kinetics, activate dead sulfur and dead lithium and reduce the loss of active substances, thereby improving the performance of the battery.
Owner:UNIV OF SCI & TECH OF CHINA
Active hydrogen tolerant catalyst, preparation method thereof and ultralow-molecular-weight poly(carbonate-ether) polyol
The invention provides an active hydrogen tolerant catalyst shown as a formula (I). The invention also provides a preparation method of ultralow-molecular-weight poly(carbonate-ether) polyol, which comprises: carrying out chain transfer polymerization reaction on carbon dioxide, an epoxy compound and an initiator under the action of the active hydrogen tolerant catalyst or the active hydrogen tolerant catalyst prepared by the preparation method to obtain the ultralow-molecular-weight poly(carbonate-ether) polyol. The active hydrogen tolerant catalyst provided by the invention is an alternatingmulti-center Lewis acid-base pair catalyst; the catalyst has high proton tolerance, so that the catalyst is not limited by the concentration of the initiator any more and can be used for preparing the ultralow-molecular-weight carbon dioxide polyol. The molecular weight of the ultralow-molecular-weight poly(carbonate-ether) polyol is 500-1000 g / mol, and the molecular weight distribution is 1.07-1.15; a carbonate segment and an ether segment exist on a main chain at the same time, and the functionality is 2-10; and the content of byproduct cyclic carbonate is lower than 1%.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Catalytic synthesis application based on frustrated Lewis acid-base pair
InactiveCN104529796ASimple reaction conditionsEasy to operateOrganic compound preparationCarbonyl compound preparationSynthesis methodsStructural formula
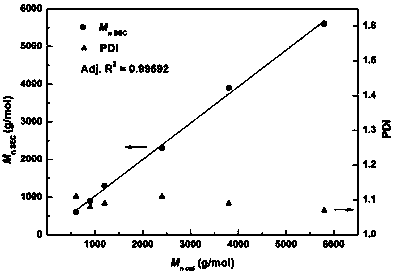
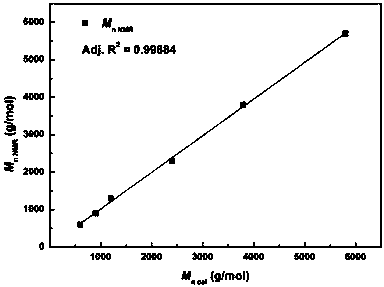
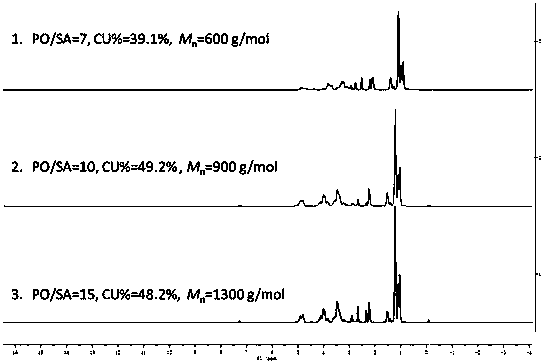
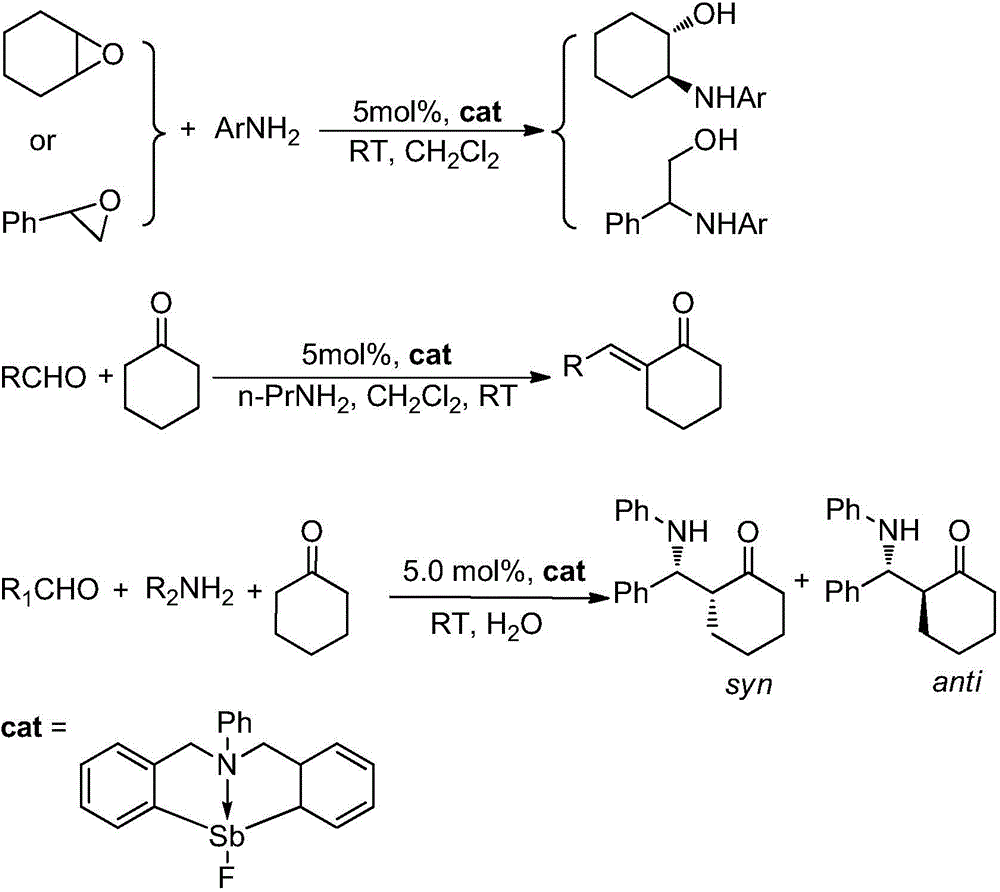
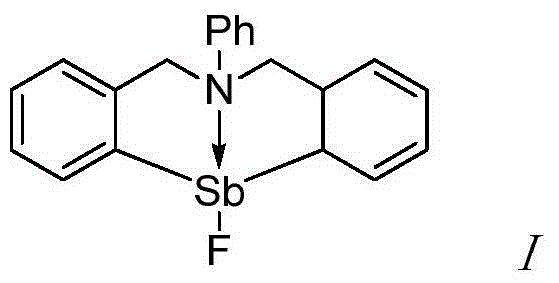
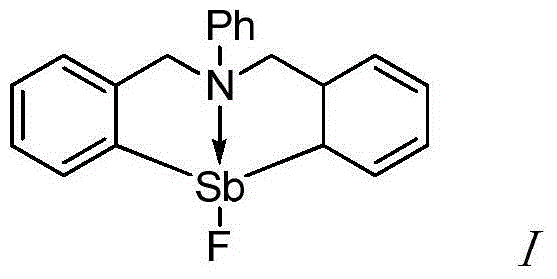
The invention provides a synthetic method for catalyzing beta-amino alcohol, alpha, beta-unsaturated ketone and beta-aminoketone based on a frustrated Lewis acid-base pair. A novel frustrated Lewis acid-base pair I which is stable in air is adopted as a catalyst and has the structural formula of PhN(CH2C6H4)2SbF, wherein Sb atom is bonded with two carbon atoms in a ligand and also forms a coordination bond together with nitrogen atom in the ligand. The method provides an efficient and environmental-friendly way for preparing a beta-amino alcohol compound from an epoxy compound and amine, preparing an alpha, beta-unsaturated ketone compound from aldehyde and ketone and preparing beta-aminoketone from aldehyde, ketone and amine. The frustrated Lewis acid-base pair is good in catalytic performance and can be repeatedly used, both the selectivity and the productivity of target products are close to 100%, the reaction conditions are simple, the operation is easy, the cost is low and the preparation process is environmental friendly.
Owner:HUNAN UNIV
Preparation method of lanthanum-modified activated carbon and method for removing fluorine ions in water
InactiveCN112263990AImprove adsorption capacityOther chemical processesWater contaminantsActivated carbonPhysical chemistry
The invention relates to the field of wastewater treatment, in particular to a preparation method of lanthanum modified activated carbon and a method for removing fluorine ions in water. The method comprises the following steps: immersing activated carbon in a lanthanum salt solution with a concentration of 0.05-0.15 mol / L, adjusting a pH value to 4-12, and conducting reacting at 25-55 DEG C. As the activated carbon is subjected to lanthanum modification, lanthanum loading can be performed on the surface of the activated carbon. The metal lanthanum can increase metal cations on the surface ofthe activated carbon and improve adsorption of anion pollutants such as fluorine ions and the like. The lanthanum element is large in atomic radius and easily loses electrons of an outer layer s, 5d or 4f'; the lanthanum element has very high affinity to fluorine ions with high electronegativity; and according to the Lewis acid-base theory, La<3+> is typical Lewis acid, F<-> is typical Lewis base,and La<3+> and F<-> form coordination covalent bond combination through coordination effect. The lanthanum-modified activated carbon can effectively improve the adsorption efficiency of an activatedcarbon adsorbent on fluorine.
Owner:CHINA UNIV OF MINING & TECH (BEIJING)
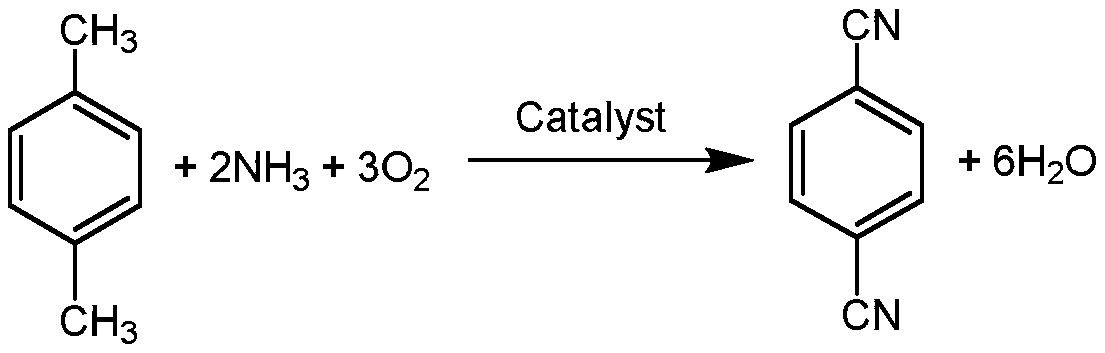
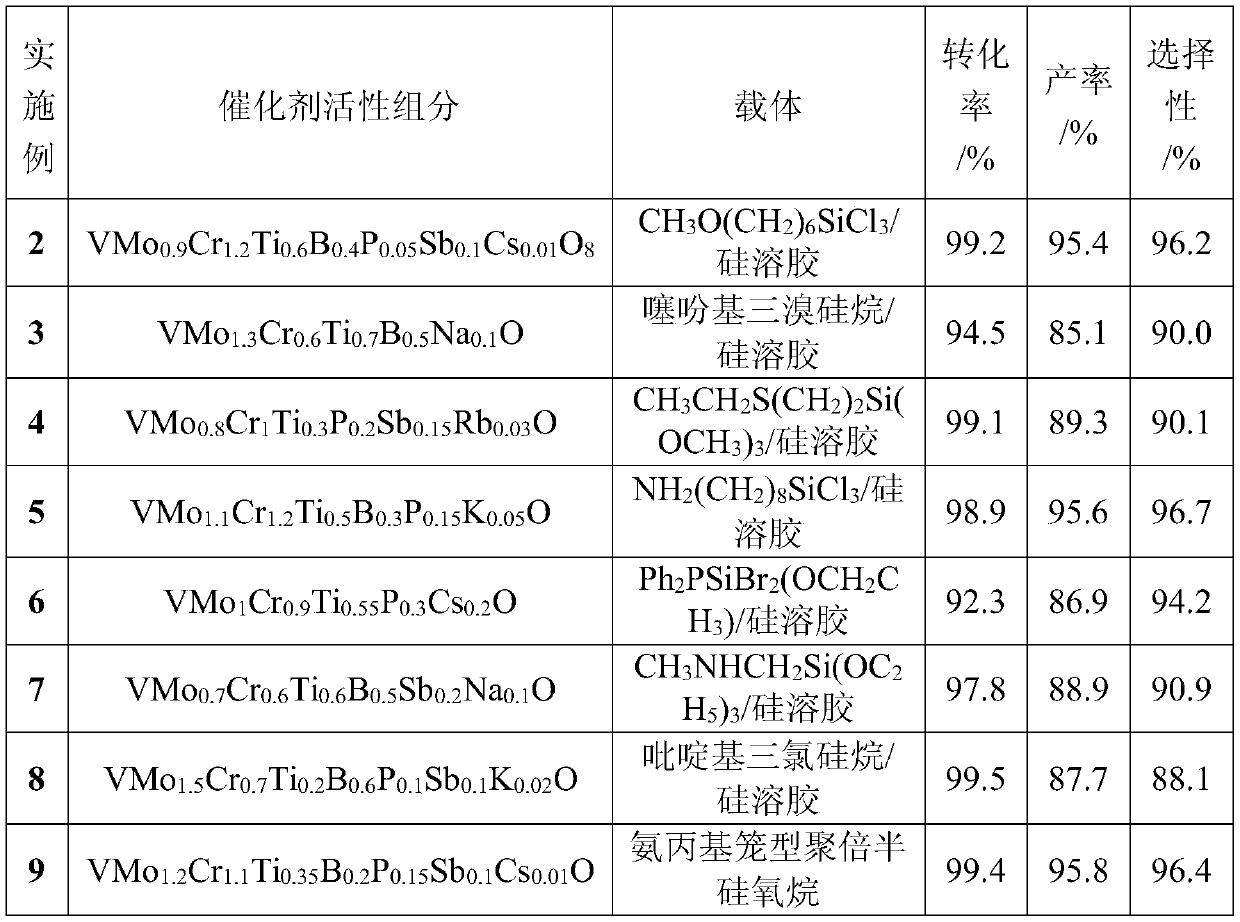
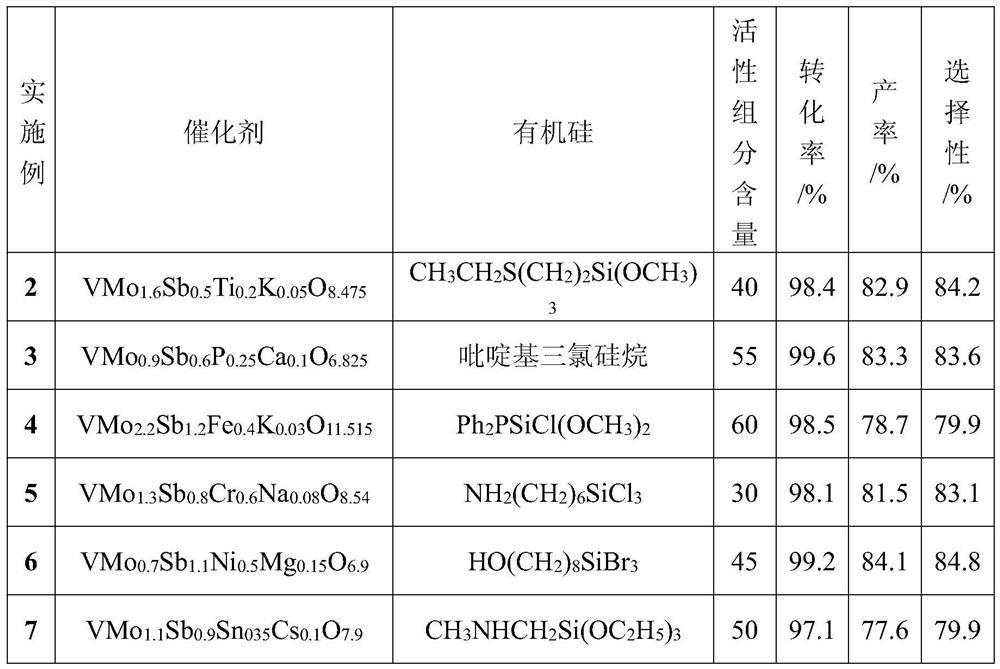
Catalyst for preparing para-Phthalonitrile by using ammoxidation method and preparation method and application
ActiveCN110227522ALow costHigh selectivityPhysical/chemical process catalystsPreparation by hydrocarbon ammoxidationFixed bedPhthalonitrile
The invention discloses a special catalyst for preparing para-Phthalonitrile by using an ammoxidation method. Silica sol is adopted as a carrier of the special catalyst, the catalyst comprises an active component of an inorganic composite oxide with at least six of vanadium, molybdenum, chromium, boron, titanium, phosphorus, antimony and an alkali metal, the active component of the catalyst is shown in a formula VMoaCrbTicBdPeSbfMgOx, in the formula, x represents the alkali metal, and the alkali metal is sodium, potassium, rubidium or caesium. The invention furthermore discloses a preparationmethod and application of the catalyst. According to the catalyst, groups with lone electron pairs on silicon and inorganic elements are subjected to a Lewis acid-alkali reaction, so that functions ofinorganic oxides with carriers are enhanced; and meanwhile, the inorganic oxides can be uniformly dispersed, the catalyst is small in component loss, high in catalytic activity and good in selectivity, and the service life of the industrial catalyst is prolonged to two years or longer from one year. The catalyst is simple in preparation method, good in thermal stability and high in mechanical strength, and can be applied to fixed bed reactors and fluidized bed reactors.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Method for catalyzing vinyl monomer polymerization by using hindered Lewis acid-base pairs based on binuclear aluminum Lewis acid
The invention discloses a method for catalyzing vinyl monomer polymerization by using hindered Lewis acid-base pairs based on binuclear aluminum Lewis acid, which belong to the technical field of polymer synthesis. According to the method, the vinyl monomer is efficiently catalyzed under mild conditions, the molecular weight and molecular weight distribution of the polymer are controllable, and anew strategy and the method are provided for controllable synthesis of the polymer. The polymerization mechanism in the invention relates to conjugate addition under the synergistic catalytic action of binuclear aluminum. The binuclear aluminum catalytic system disclosed by the invention has the advantages of low cost, easiness in obtaining, convenience in operation, mild reaction conditions, widemonomer adaptability and the like. In addition, efficient conversion of different monomers can be achieved by adjusting pKa of Lewis acid / base, the molecular weight and molecular weight distributionof the polymer can be accurately regulated and controlled, and the initiation efficiency is close to 100%. The catalytic system can efficiently catalyze homopolymerization and copolymerization reactions of methyl methacrylate and butyl acrylate, and also provides a new synthesis strategy and approach for copolymer thermoplastic elastomers of methyl methacrylate and butyl acrylate.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Precursor solution for organic polymer film formation and method for forming organic polymer film
InactiveUS20050234157A1Increase crosslink densityLow dielectric constantPlastic/resin/waxes insulatorsSemiconductor/solid-state device manufacturingAcid–base reactionOrganic polymer
A precursor solution for use in forming an organic polymer film includes a first monomer which is a Lewis acid, a second monomer which is a Lewis base to be brought into a Lewis acid-base reaction, and a sacrificial organic molecule including a polar group.
Owner:PANASONIC CORP
Method for preparing amphiphilic block copolymer through cooperation of light-operated free radical polymerization and ring-opening copolymerization
The invention discloses a method for preparing an amphiphilic block copolymer through cooperation of light-operated free radical polymerization and ring-opening copolymerization, which comprises the following steps: simultaneously adding a Lewis acid-base pair catalytic system consisting of a reversible addition chain transfer reagent containing hydroxyl or carboxyl, a photoinitiator, a hydrophilic vinyl monomer, a Lewis acid-base pair system composed of Lewis acid and Lewis base, an epoxy compound, and a cyclic acid anhydride. The chain transfer reagent is used as a ring-opening polymerization initiator, and a Lewis acid-base pair is used as a catalyst to catalyze ring-opening copolymerization of cyclic anhydride and epoxide, so that the chain transfer agent is attached to the tail end of polyester. Meanwhile, visible light or ultraviolet light is irradiated, the photoinitiator is decomposed to generate free radicals, free radical polymerization of allyl monomers is achieved, and hydrophilic polyolefin chains and polyester chains are subjected to addition through reversible addition of a chain transfer agent, so that the amphiphilic block polymer is formed. The preparation method is simple and easy to operate, the production cost is low, certain biodegradability is achieved, the polymerization process is easy to control, and the product structure can be accurately regulated and controlled.
Owner:ANYANG INST OF TECH
Lewis acid-base pair catalyst, preparation method and method for catalytically synthesizing polyester
The invention relates to the field of catalyst synthesis. Aiming at the problems of lack of existing catalyst types as well as low polymer molecular weight, low initiation efficiency, existence of side reaction and low monomer conversion rate in polyester generation through phthalic anhydride and cyclohexene oxide bulk copolymerization, the invention provides a Lewis acid-base pair which has a structure shown in the specification, wherein n is equal to 1, 2, 3 or 4, and X is equal to Cl, Br or I. The preparation method of the Lewis acid-base pair catalyst comprises the following steps of: preparing quaternary phosphonium salt from triphenylphosphine and halogenated olefin; and preparing the catalyst from the quaternary phosphonium salt and 9-boron bicyclo [3.3.1] nonane. The invention also provides a method for synthesizing polyester, which comprises the step of: by taking anhydride and epoxy monomer as raw materials, carrying out ring-opening polymerization under the catalysis of the Lewis acid-base pair catalyst to generate an alternating copolymer. The catalytic system has the advantages of simplicity in preparation, high activity, convenience in use, low cost and wide applicability, and is very suitable for industrial production.
Owner:QINGDAO UNIV OF SCI & TECH
Compounds and compositions for coating glass with silicon oxide
InactiveUS7897259B1Semiconductor/solid-state device manufacturingCeramic layered productsGas phaseSilicon oxide
Silicon compounds useful as coating reactants for the chemical vapor deposition of silicon oxide are disclosed, along with compounds useful as accelerants to increase the deposition rate of silicon oxide. The silicon-containing precursor comprises the structural formulawherein R1 is an alkyl, alkenyl, alkynyl or aryl radical which may be substituted, and R2 is a functional group which increases the reactivity of the silicon compound by withdrawing electron density away from the silicon atom, such as hydrogen, halogen, alkenyl, alkynyl, halogenated alkyl and perhalogenated alkyl radicals. The accelerant is a compound selected to take advantage of the partial positive charge on the silicon atom. Such accelerant compounds include Lewis acids and bases; water; ozone; trivalent compounds of nitrogen, boron and phosphorus; tetravalent compounds of sulfur and selenium; pentavalent compounds of phosphorus and a variety of metal compounds. Also disclosed are compositions including an additional metal-containing coating precursor, such as an organotin compound, to deposit another metal oxide along with silicon oxide.
Owner:VITRO FLAT GLASS LLC
Emodin solid dispersion as well as preparation method and application thereof
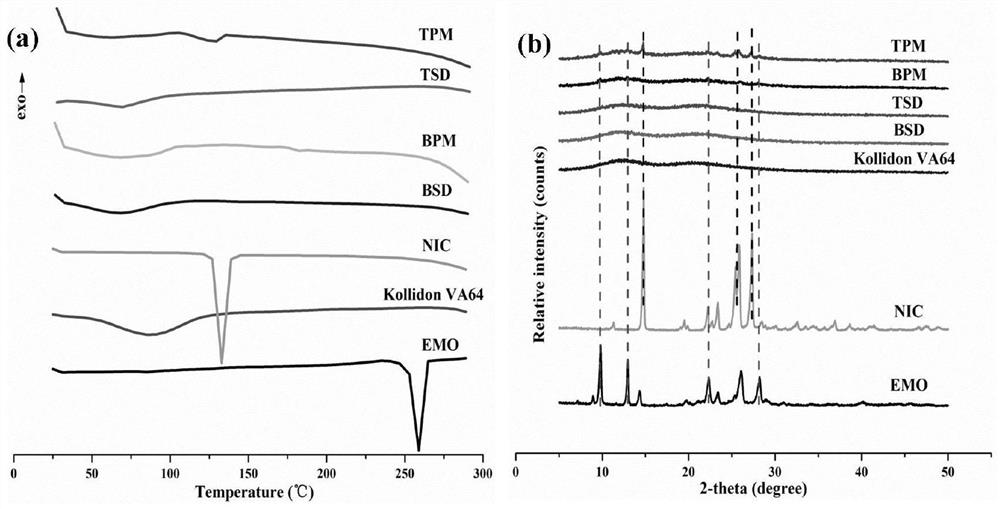
ActiveCN113616599AGood effectImprove solubilityAntibacterial agentsOrganic active ingredientsOrganic solventFree energies
The invention belongs to the technical field of medicines, and particularly discloses an emodin solid dispersion as well as a preparation method and application thereof. The emodin solid dispersion disclosed by the invention is obtained by mixing emodin, a high polymer carrier and a bonding agent and then treating through a hot melt extrusion technology, and the drug loading capacity of the emodin solid dispersion is 5-20%. Through Lewis acid-base and hydrogen-bond interaction, an emodin molecular dimer structure is broken, so that an amorphous drug is in a supersaturated state; meanwhile, the emodin solid dispersion system is relatively low in Gibbs free energy and molecular mobility and relatively strong in intermolecular interaction, so that the physical stability of the amorphous preparation is enhanced, and the in-vitro dissolution of the amorphous preparation is prolonged; according to the emodin solid dispersion and the preparation method thereof, no organic solvent is used, the preparation is safe and efficient, the obtained emodin solid dispersion improves the dissolution rate and solubility of insoluble drugs, the drug loading capacity is high, the stability is good, a good intermediate is provided for other oral solid preparations of emodin, and the preparation process is simple, efficient, safe and suitable for industrial production.
Owner:NINGXIA MEDICAL UNIV
Application of Lewis acid-base pair in polymerization-induced self-assembly, fiber morphology amphiphilic block polymer as well as preparation method and application of fiber morphology amphiphilic block polymer
ActiveCN114163592AUniform diameterHigh catalytic activityTransportation and packagingMixingMethacrylatePolymer science
The invention provides application of a Lewis acid-base pair in polymerization-induced self-assembly, a fiber morphology amphiphilic block polymer as well as a preparation method and application of the fiber morphology amphiphilic block polymer, and relates to the technical field of liquid crystal polymers. The invention provides an application of a Lewis acid-base pair in polymerization induced self-assembly. The Lewis acid-base pair comprises Lewis acid and Lewis base. According to the invention, a polymerization-induced self-assembly method is adopted, a Lewis acid-base pair is used as a catalyst, and the catalyst has high catalytic activity on a methacrylate monomer, so that the time of a polymerization-induced self-assembly reaction can be remarkably shortened; the amphiphilic block polymer has good polymerization controllability on a polymerization induced self-assembly system, and the amphiphilic block polymer with uniform and adjustable fiber diameter can be prepared by controlling the ratio of the stable chain segment monomer to the nucleation chain segment monomer; according to the preparation method provided by the invention, the size-adjustable amphiphilic block polymer with the fiber morphology can be efficiently and rapidly synthesized through one-pot two-step, and the operation is simple.
Owner:JILIN UNIV
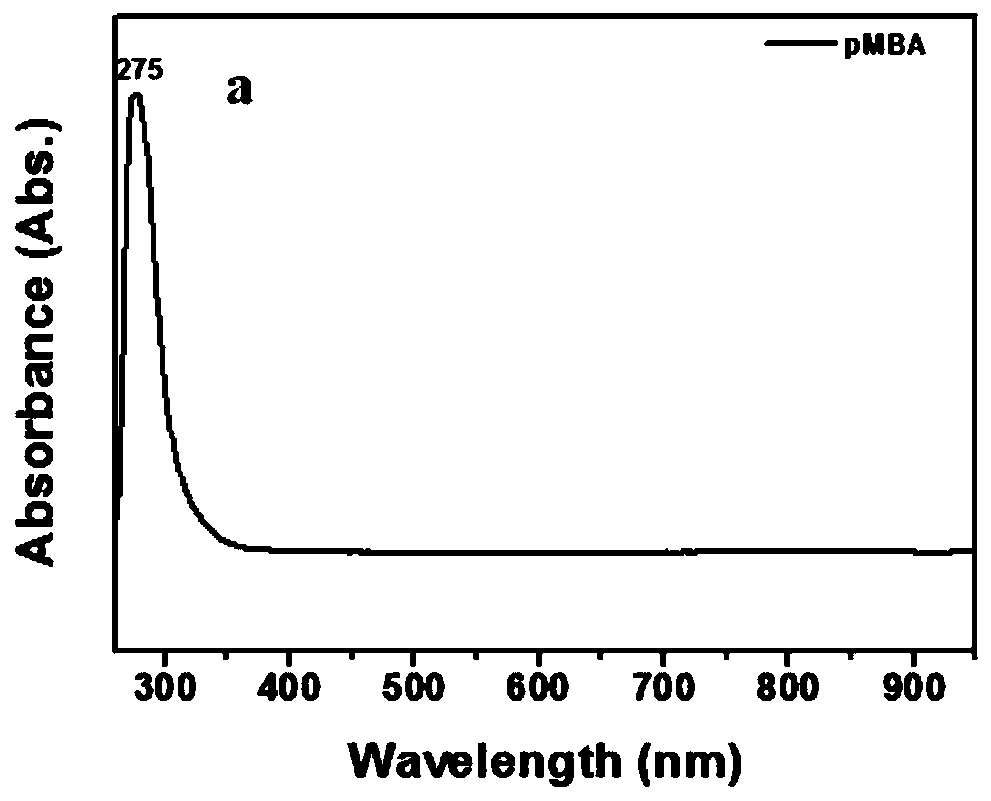
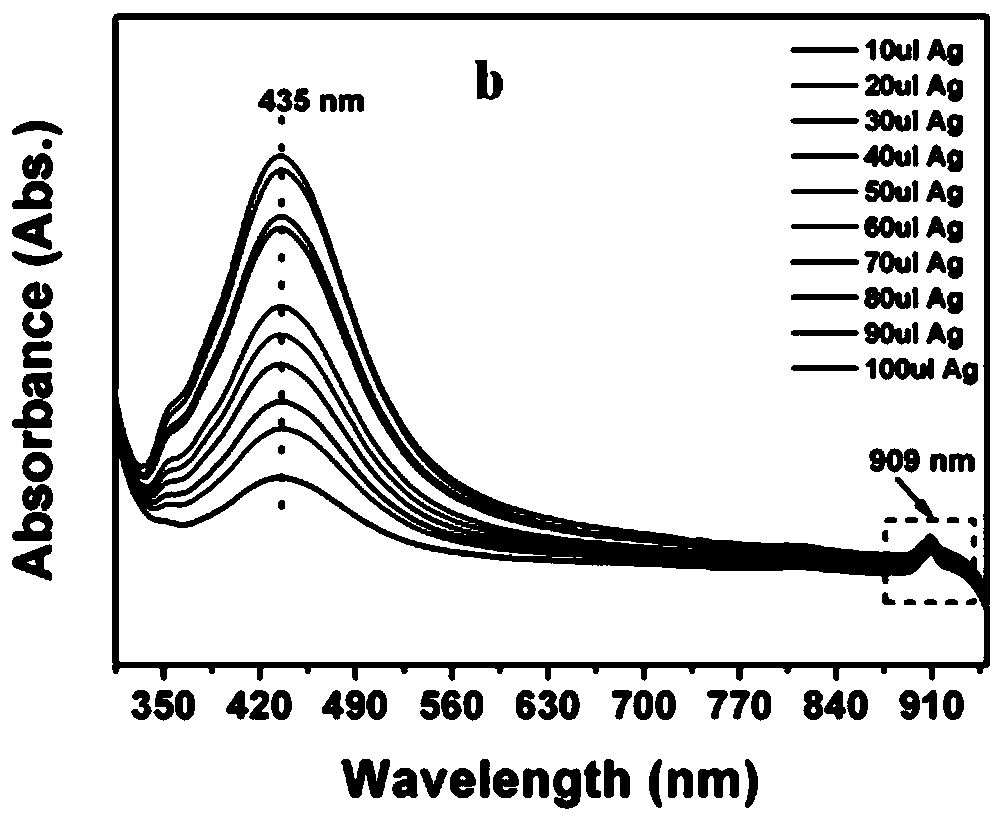
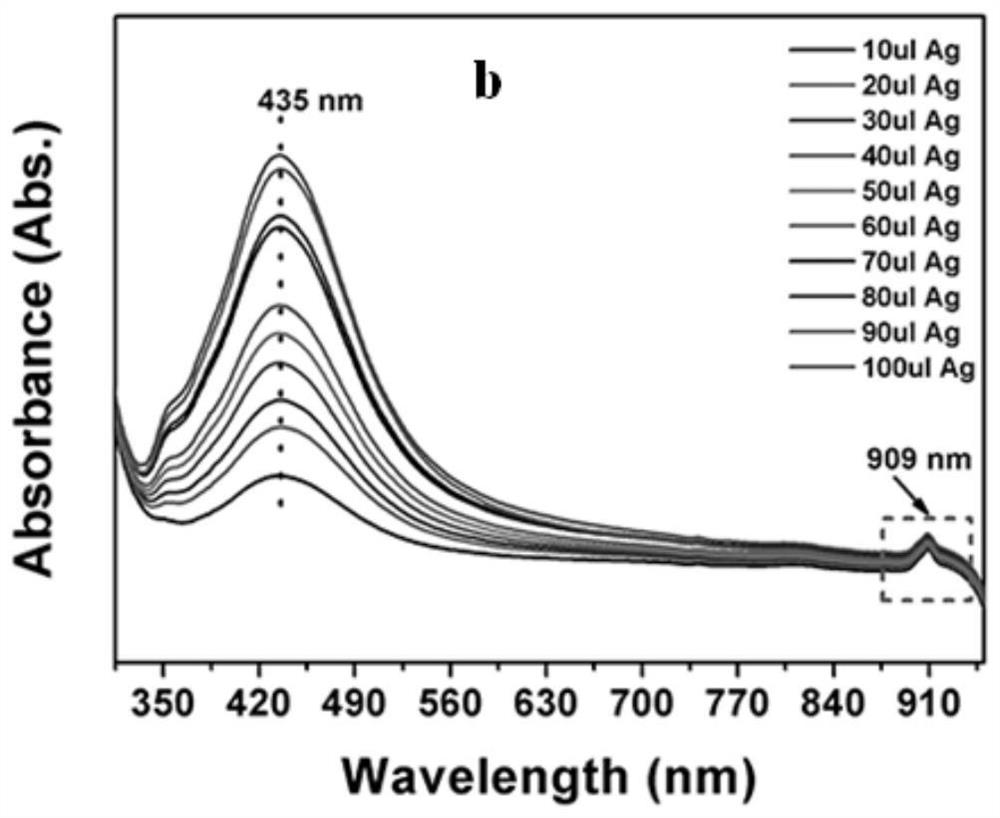
Method for promoting ionization of benzoic acid derivatives based on Ag NPs
ActiveCN110698371AIonization reaction promotesIonization reaction promotionThiol preparationMetal/metal-oxides/metal-hydroxide catalystsBenzoic acidPtru catalyst
The invention relates to a method for promoting ionization of benzoic acid derivatives based on Ag NPs. According to a technical scheme in the invention, the method comprises the following steps: dissolving benzoic acid derivatives in absolute ethyl alcohol, then adding an Ag NPs catalyst, and carrying out an ionization reaction under illumination. According to the invention, alkali is not into asystem of the invention as in the prior art; however, an ionization degree can be clearly seen to increase gradually, so the lewis acid-base racemate--Ag NPs fully plays the role of an alkali, and more importantly, the enhancement effect of the lewis acid-base racemate--Ag is far higher than that of basic alkali. In addition, a surface enhanced Raman spectrum technology is introduced, and the system is monitored through such a simple and efficient means. Moreover, the flow direction of electrons is defined. More importantly, the generation of hot electrons is regulated and controlled based onthe electron withdrawing effect, so the system is promoted to generate more hot electrons and realizes a reaction which cannot conventionally occur in organic chemistry.
Owner:LIAONING UNIVERSITY
Special catalyst for preparing isophthalonitrile by ammoxidation reaction, preparation method and application
ActiveCN109876794BLow costHigh selectivityPreparation by hydrocarbon ammoxidationMetal/metal-oxides/metal-hydroxide catalystsXylylenePtru catalyst
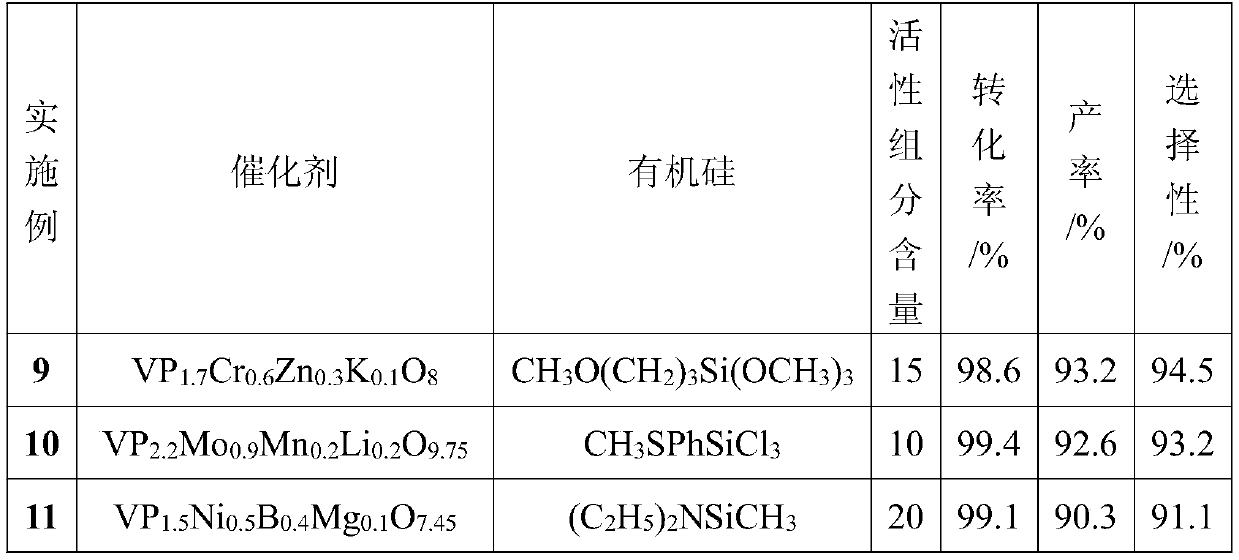
The invention discloses a special catalyst for preparing isophthalonitrile by ammoxidation of m-xylene. The carrier is silica gel, the main catalyst is three components of V, Mo and Sb, and the co-catalyst is at least one of D and E components. species; its active components are expressed as: VMo b Sb c D. d E. e o x ; The D is boron, chromium, titanium, phosphorus, nickel, bismuth, manganese, iron, cobalt, copper, zinc or tin; E is potassium, lithium, sodium, cesium, magnesium or calcium. The invention also discloses the preparation method and application of the catalyst. In the present invention, groups containing lone electron pairs on silicon are used to carry out Lewis acid-base reaction with inorganic elements, thereby strengthening the effect of inorganic oxides and supports; at the same time, the dispersion of inorganic oxides is more uniform, the loss of catalyst components is small, and the catalytic activity is high. , good selectivity, prolonging the service life of industrial catalysts from one year to more than two years. The preparation method of the catalyst is simple, the thermal stability and mechanical strength are good, and the catalyst can be used in fixed-bed and fluidized-bed reactors.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
TiO2-x-based hindered Lewis acid-base pair photocatalyst as well as preparation method and application thereof
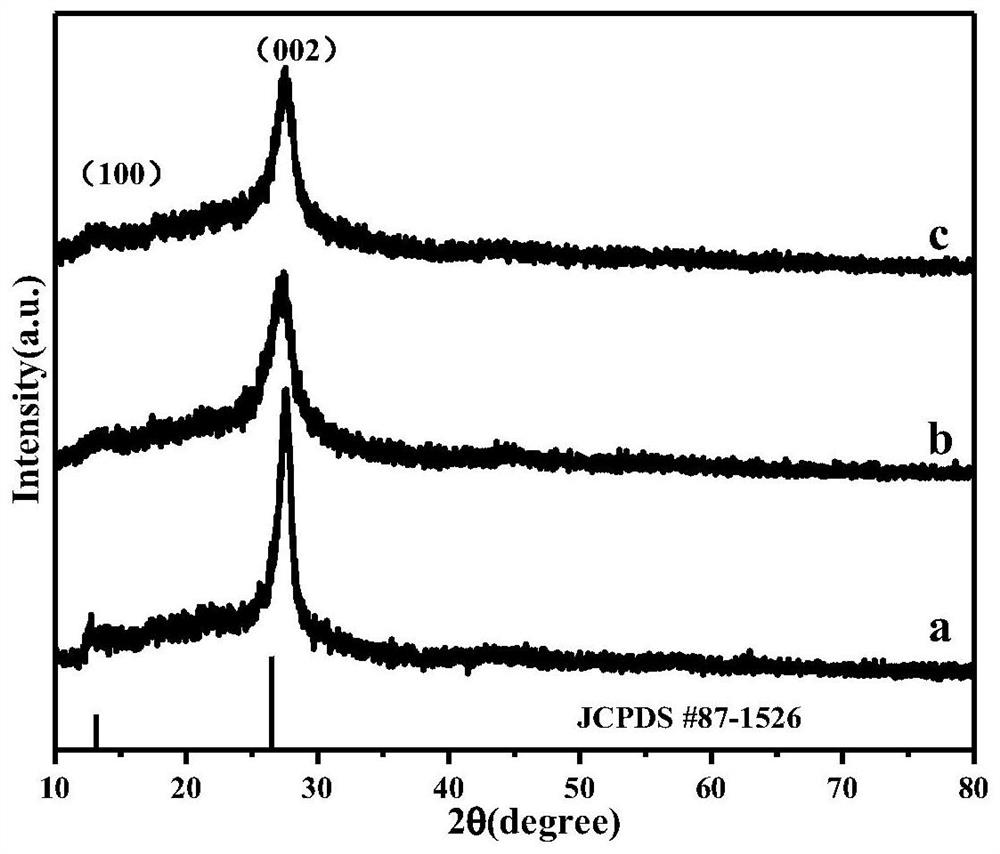
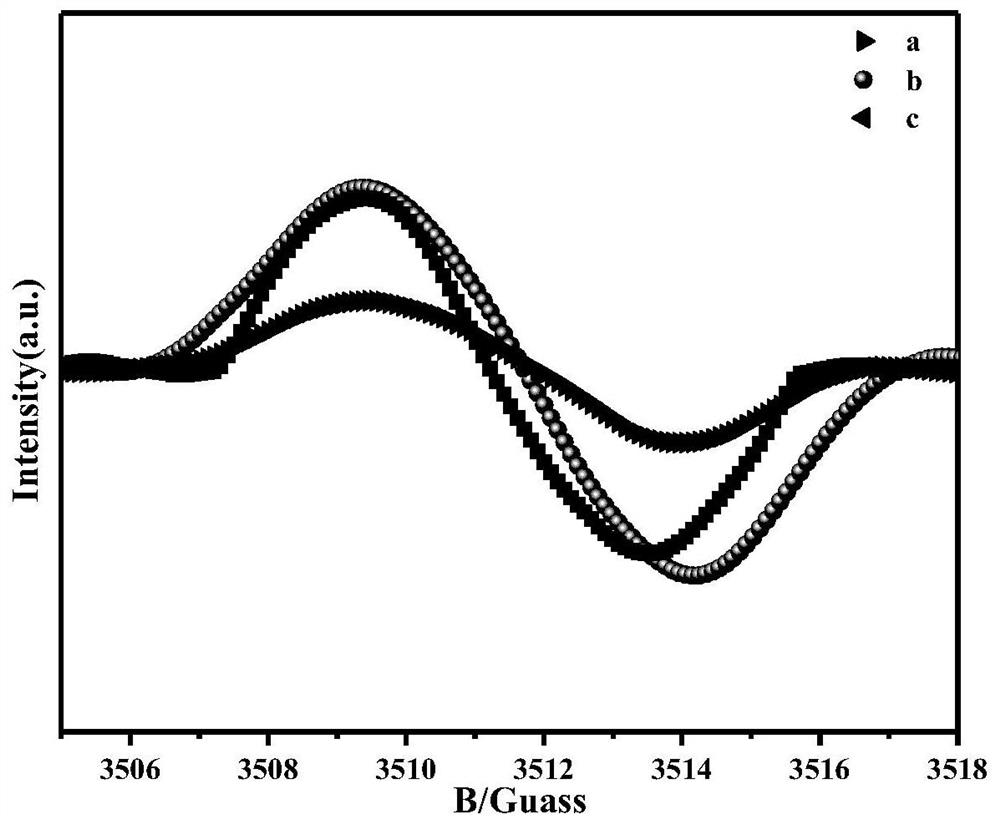
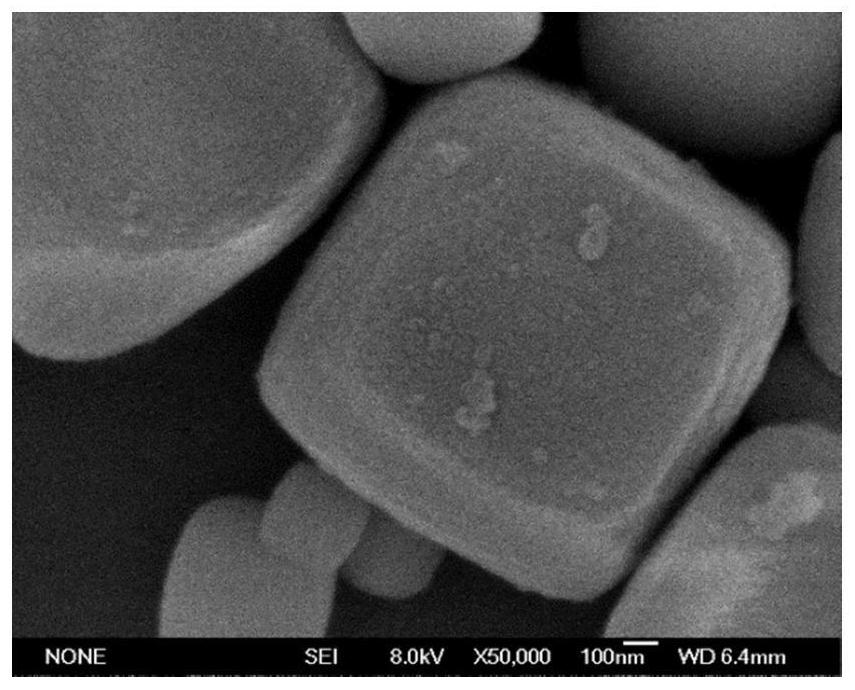
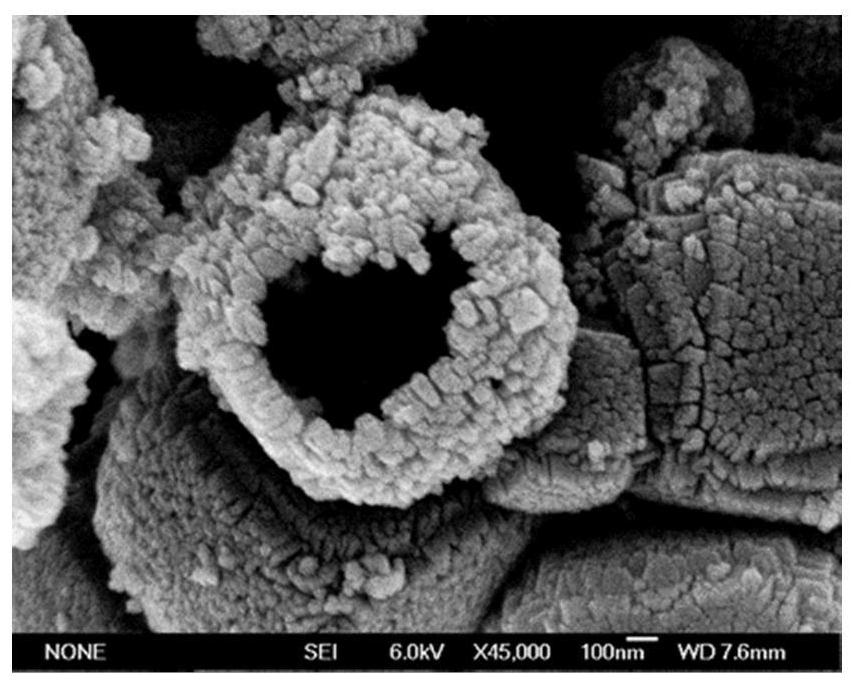
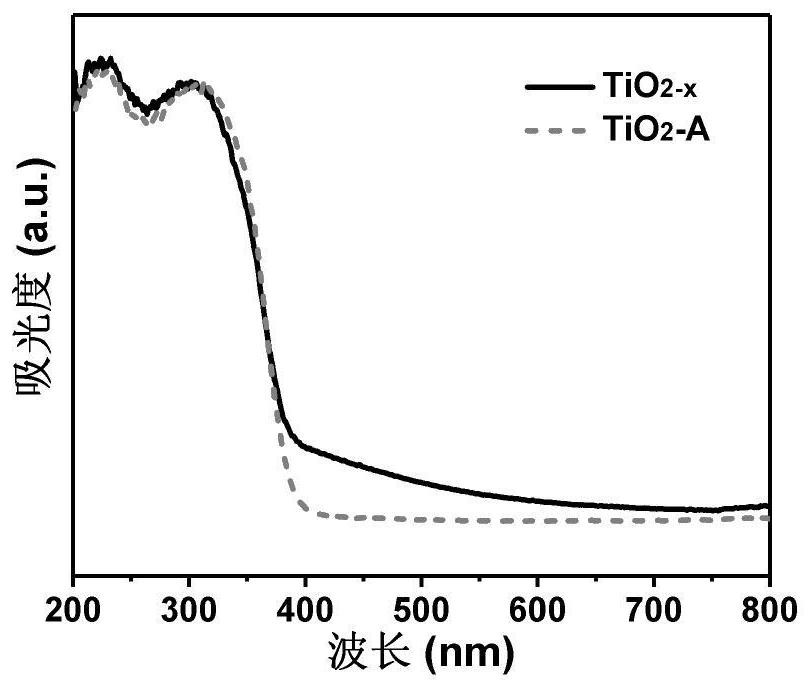
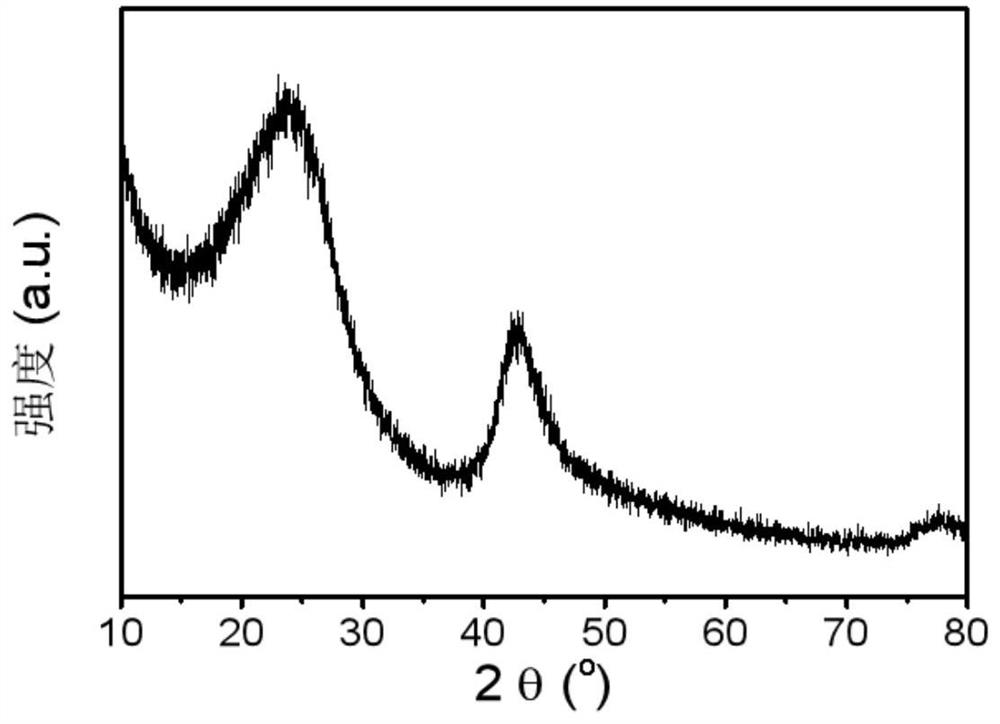
ActiveCN112569919AImprove adsorption capacityImprove reduction efficiencyEnergy inputCatalyst activation/preparationPtru catalystOxygen vacancy
The invention belongs to the field of material synthesis and catalytic reaction, and particularly relates to a TiO2x-based hindered Lewis acid-base pair photocatalyst as well as a preparation method and application thereof. Perovskite MTiO3 with a certain structure is synthesized firstly, and then MTiO3 is subjected to in-situ topological transformation and chelation to remove M2+; thus, the photocatalyst containing oxygen vacancies and hindered Lewis acid-base pairs with adjacent hydroxyl groups on the surface is obtained. The TiO2x-based photocatalyst has relatively high CO2 adsorption capacity, and can realize adsorption and activation of gas molecules, so that the CO2 reduction efficiency of the TiO2-based photocatalyst is improved. The preparation method of the catalyst is simple andeasy to implement and mild in reaction condition and has wide application prospects in the aspects of reducing the content of CO2 in the atmosphere and efficiently utilizing solar energy to achieve chemical energy conversion. The technical scheme is a developable novel method for constructing hindered Lewis acid-base pairs, and can be used for efficient photocatalytic CO2 reduction reaction.
Owner:QINGDAO UNIV OF SCI & TECH
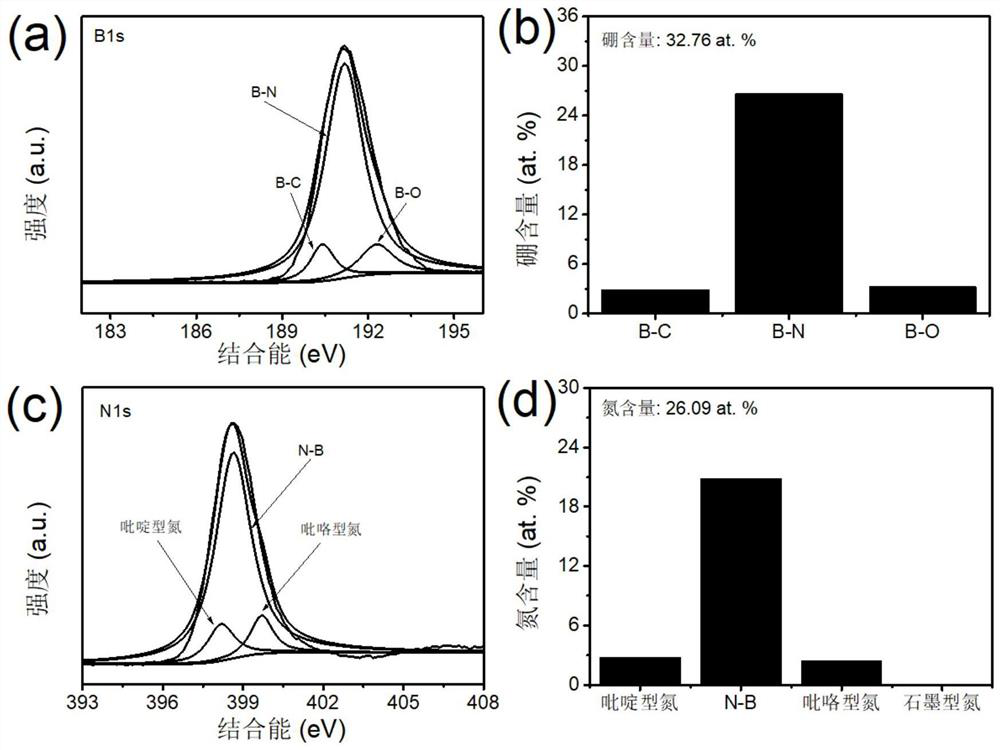
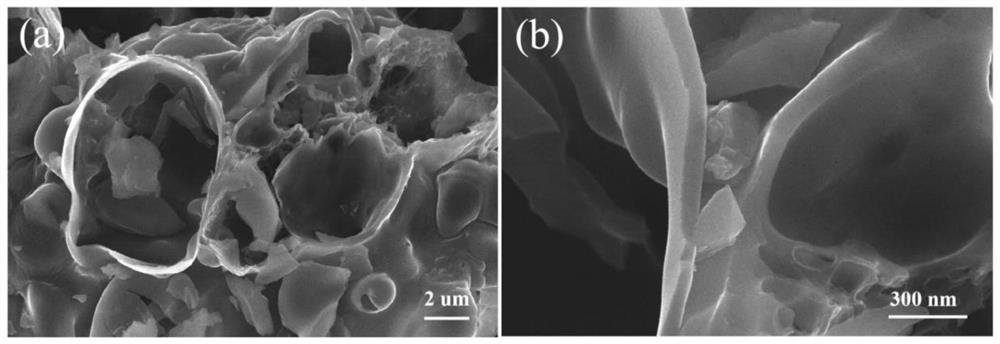
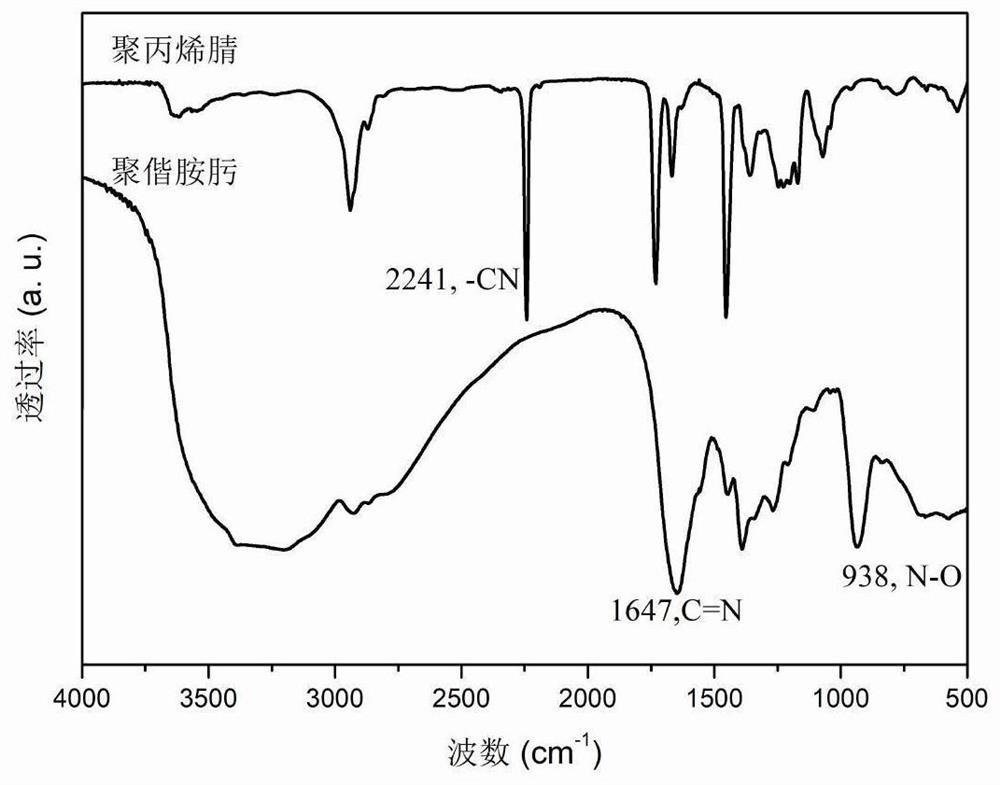
Porous carbon material with B-N Lewis acid-base pair structure as well as preparation method and application of porous carbon material
PendingCN113813926APrecise adsorptionLow equipment requirementsGas treatmentOther chemical processesPorous carbonSorbent
The invention provides a porous carbon material with a B-N Lewis acid-base pair structure and a preparation method and application of the porous carbon material. The surface of the porous carbon material is provided with the B-N Lewis acid-base pair structure, and the preparation method comprises the steps that a nitrogen supply source, L-cysteine and a boron supply source are mixed and ground to obtain a solid mixture precursor; and the solid mixture precursor is subjected to two-step calcination to obtain the porous carbon material with the B-N Lewis pair structure. The obtained porous carbon material is used as a formaldehyde adsorbent or a formaldehyde catalytic oxidant substrate. The formaldehyde special-effect adsorbent with B-N Lewis acid-base para-sites, high specific surface area and porous characteristics is obtained through a simple and convenient preparation process, and the formaldehyde special-effect adsorbent has very high removal efficiency on formaldehyde in the living environment and also has good application potential in the fields of rare earth industrial sewage treatment, electro-catalysis and the like.
Owner:JIANGXI INST OF RARE EARTHS CHINESE ACAD OF SCI
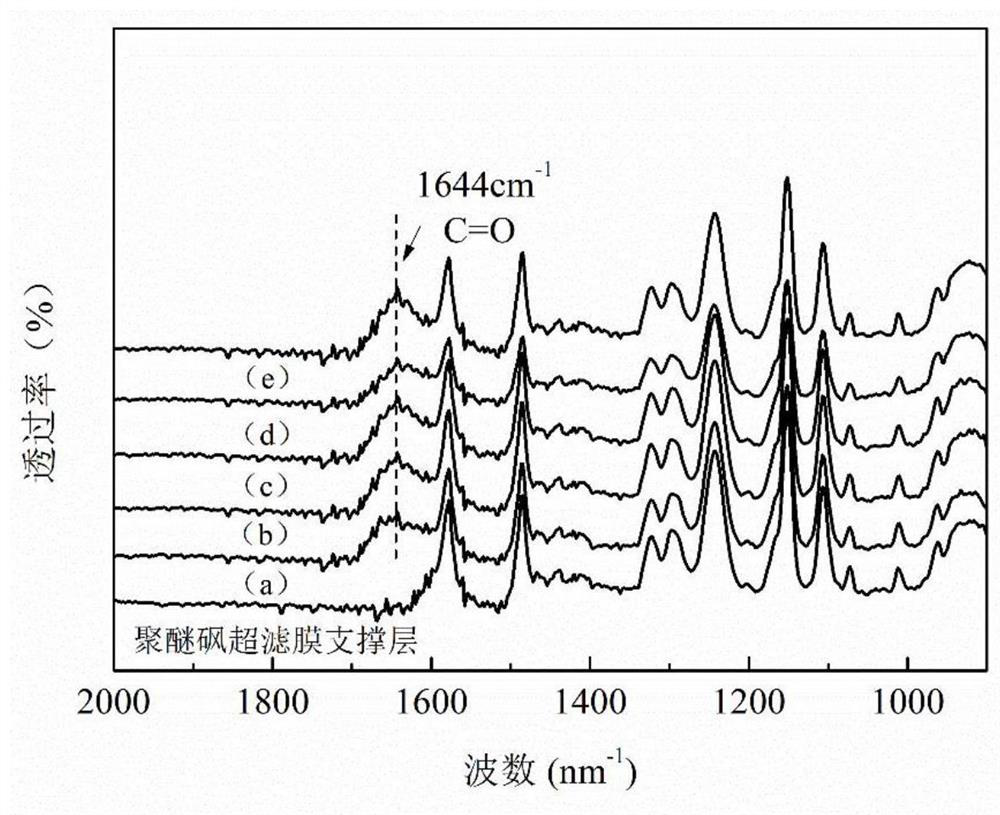
Preparation method of nanofiltration membrane based on polyamidoxime as boundary layer
ActiveCN113413776AHigh electronegativityImprove hydrophilicityWater/sewage treatment bu osmosis/dialysisReverse osmosisElectronegativityOil phase
The invention provides a preparation method of a nanofiltration membrane based on polyamidoxime as a boundary layer, and belongs to the field of nanofiltration membrane preparation. According to the invention, the polyamidoxime rich in amidoxime groups is used as an organic boundary layer, so that the hydrophilicity of the surface of a supporting layer can be obviously enhanced, the uniform spreading of a water solution of a water-phase monomer piperazine on the boundary layer is facilitated, a compact and thin PA layer with small surface aperture is prepared, and the polyamidoxime can generate hydrogen-bond interaction and Lewis acid-base interaction with piperazine, the diffusion of piperazine to an oil phase solution is effectively inhibited, the instability of interfacial polymerization is caused, a wrinkled PA layer with relatively strong electronegativity on the surface is formed, the effective permeation area is further remarkably increased, the permeation transmission channels of the finally obtained nanofiltration membrane are increased and the transmission resistance is reduced due to the reduction of the thickness of the PA layer and the increase of the effective permeation area of the PA layer, and the permeation flus is greatly improved. Meanwhile, the surface of the PA layer has relatively strong electronegativity and relatively small surface aperture, so that the solute rejection rate is increased.
Owner:EAST CHINA UNIV OF TECH
Amphiphilic block polymer nanoparticles with different morphologies as well as preparation method and application of amphiphilic block polymer nanoparticles
The invention provides amphiphilic block polymer nanoparticles with different morphologies as well as a preparation method and application thereof, belonging to the technical field of polymers. Under the catalytic action of a Lewis acid-base pair, an acrylate monomer stable chain segment monomer and a nucleation chain segment monomer are subjected to a polymerization reaction, and the amphiphilic block polymer nanoparticles with different morphologies are obtained; and the nucleating chain segment monomer comprises trifluoroethyl methacrylate or benzyl methacrylate. According to the invention, the Lewis acid-base pair is used as a catalyst, so highly asymmetric amphiphilic block polymer nanoparticles with different morphologies can be efficiently synthesized through a one-pot one-step method, and the polymer nanoparticles are clear in structure, few in monomer insertion errors, good in polymerization controllability and narrow in molecular weight distribution. The amphiphilic block polymer nanoparticles with spherical morphology, worm morphology and vesicle morphology can be obtained by controlling the dosage ratio of the two monomers.
Owner:JILIN UNIV
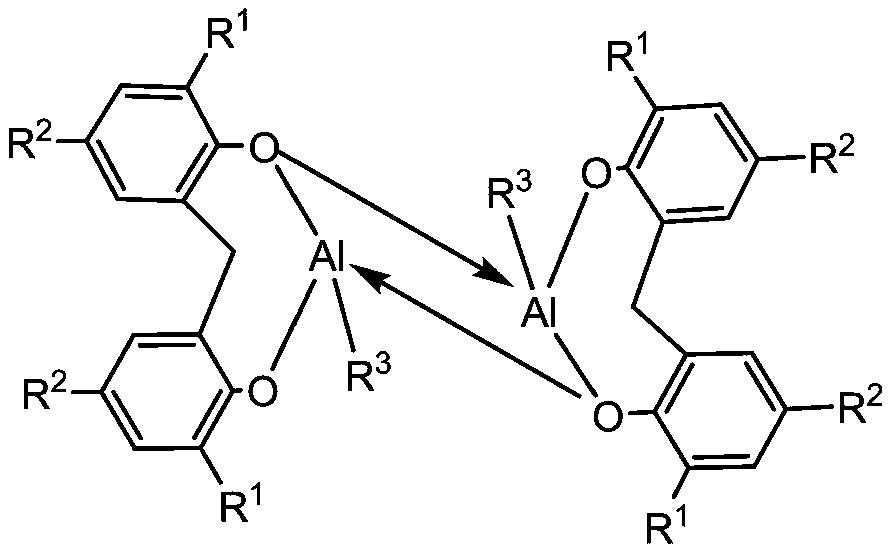
Lewis acid-base pair catalytic initiator and application thereof
ActiveUS20210316287A1Maximizing atomic economyReduce probabilityOrganic-compounds/hydrides/coordination-complexes catalystsArylCarbon number
The present disclosure provides a Lewis acid-base pair catalytic initiator and an application thereof. The Lewis acid-base pair catalytic initiator includes a Lewis acid and a Lewis base, the Lewis acid having a structural general formula as shown in formula (I) and the Lewis base having a structural general formula as shown in formula (II); wherein: the A is selected from element Baron or element Aluminum; the R1, R2, R3, R4 are independently selected from alkyl, alkoxy, aryl or halogen groups; the alkyl or alkoxy have a carbon number being equal to or greater than 1 to equal to or less than 16; the aryl contains substituents with the number being equal to or less than 5, the substituents being selected from methyl, methoxy or halogen; n is selected from an integer from 1 to 16.
Owner:ZHEJIANG UNIV
Lewis acid-base pair catalytic initiator and application thereof
ActiveUS11247199B2Reduce probabilityReduce rateOrganic-compounds/hydrides/coordination-complexes catalystsArylCarbon number
The present disclosure provides a Lewis acid-base pair catalytic initiator and an application thereof. The Lewis acid-base pair catalytic initiator includes a Lewis acid and a Lewis base, the Lewis acid having a structural general formula as shown in formula (I) and the Lewis base having a structural general formula as shown in formula (II); wherein: the A is selected from element Baron or element Aluminum; the R1, R2, R3, R4 are independently selected from alkyl, alkoxy, aryl or halogen groups; the alkyl or alkoxy have a carbon number being equal to or greater than 1 to equal to or less than 16; the aryl contains substituents with the number being equal to or less than 5, the substituents being selected from methyl, methoxy or halogen; n is selected from an integer from 1 to 16.
Owner:ZHEJIANG UNIV
Method for synthesizing fluorescent functional polyester-based amphiphilic polymer
The invention discloses a method for synthesizing polyunsaturated side group-containing polyester and preparing a fluorescent functional amphiphilic polymer through post-modification of the polyunsaturated side group-containing polyester. Fluorescent functional small organic molecules such as rhodamine and the like are used as initiators, an organoboron compound is used as lewis acid and organic alkali to form different lewis acid-base pair catalytic systems, ring-opening copolymerization of cyclic anhydride and epoxide is catalyzed, and polyunsaturated side group-containing completely alternating polyesters with different molecular weights are prepared. Post-modification is conducted on unsaturated double bonds of side groups through a mercapto-alkene click reaction, and hydrophilic side groups or side chains are introduced, so as to prepare the fluorescent functional amphiphilic polymer. The catalyst is cheap and easy to obtain, the initiator is selected according to the requirement of fluorescence characteristics, different types of amphiphilic polymers can be obtained by adjusting the types of cyclic anhydride, epoxide and hydrophilic side groups, no metal is introduced in the preparation process, the main chain structure of the product is polyester, and the advantages of low toxicity, biodegradability and excellent biocompatibility are achieved.
Owner:ANYANG INST OF TECH
Lewis acid-base bifunctional catalyst and preparation method and application thereof
ActiveCN112473743AStable 3D frame structureLarge specific surface areaCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystCatalytic oxidation
The invention relates to a Lewis acid-base bifunctional catalyst and a preparation method and application thereof, the catalyst is a catalyst with a three-dimensional porous structure assembled by a mixed ligand consisting of 2, 4, 6-tri (4pyridyl)-1, 3, 5-triazine and trimesic acid and Ni (II) serving as a central ion. Compared with the prior art, the catalyst material provided by the invention has a large specific surface area and Lewis acid-base bifunctional active sites, can be used for a one-pot series aromatic alcohol catalytic oxidation Knoevenagel condensation reaction under mild conditions, and has the highest catalytic efficiency of 87% and the selectivity of 99%.
Owner:SHANGHAI INST OF TECH
Amphiphilic block polymer nanoparticles with different morphologies and their preparation methods and applications
The invention provides amphiphilic block polymer nanoparticles with different morphologies, a preparation method and application thereof, and relates to the technical field of polymers. In the present invention, under the catalysis of Lewis acid-base pair, the acrylate monomer stabilizes the segment monomer and the nucleation segment monomer is polymerized to obtain amphiphilic block polymer nanoparticles with different morphologies; Nucleating segment monomers include trifluoroethyl methacrylate or benzyl methacrylate. The Lewis acid-base pair used in the invention can efficiently synthesize highly asymmetric amphiphilic block polymer nanoparticles with different morphologies through a "one-pot, one-step" method as a catalyst, and the polymer nanoparticles have a clear structure, and the monomers Less mis-insertion, good polymerization control and narrow molecular weight distribution. Amphiphilic block polymer nanoparticles with spherical, worm and vesicle morphologies can be obtained by controlling the dosage ratio of the two monomers.
Owner:JILIN UNIV
Heterogeneous hindered Lewis pair catalyst as well as preparation method and application thereof
PendingCN113058646AThe synthesis process is simpleImprove toleranceOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbon by hydrogenationPtru catalystBoronic acid
The invention discloses a heterogeneous hindered Lewis pair catalyst which is prepared from an amine compound and hydroxyphenylboronic acid or hydroxyphenylboronic ester through a hydrothermal polymerization reaction, and the catalyst can efficiently catalyze selective hydrogenation of an alkyne compound to prepare a corresponding olefin compound. The catalyst is simple in preparation method, low in cost, stable, insensitive to water and high in reaction activity, and the problems that a traditional catalyst is complex in preparation process, prone to deliquescence and deactivation and the like are solved; meanwhile, the catalyst is a non-metal hydrogenation catalyst and is suitable for many metal-free catalysis scenes, and the problems of metal residues and the like are solved; in addition, the solid heterogeneous catalyst avoids the problem that the catalyst is difficult to separate from the product after the reaction, and is convenient to treat and environment-friendly.
Owner:NANJING FORESTRY UNIV
A method based on ag NPs to promote the ionization of benzoic acid derivatives
ActiveCN110698371BIonization reaction promotesIonization reaction promotionThiol preparationMetal/metal-oxides/metal-hydroxide catalystsBenzoic acidPtru catalyst
The invention relates to a method for promoting the ionization of benzoic acid derivatives based on Ag NPs. The adopted technical scheme is: dissolving benzoic acid derivatives in absolute ethanol, then adding Ag NPs catalyst, and ionizing reaction under light. In the present invention, there is no conventional addition of alkali in this system, but it can be clearly seen that the degree of ionization is gradually increasing, so the "racemate of Lewis acid-base"-Ag NPs fully exerts the role of alkali, and the more critical It is that it acts as a boost far beyond that of the base base. Moreover, the present invention introduces surface-enhanced Raman spectroscopy technology, and monitors the system through this simple and efficient means. Moreover, the present invention clarifies the flow direction of electrons. More importantly, the present invention regulates the generation of hot electrons based on the electron-withdrawing effect, prompting the system to generate more hot electrons, and realizing reactions that cannot occur in conventional organic chemistry.
Owner:LIAONING UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com