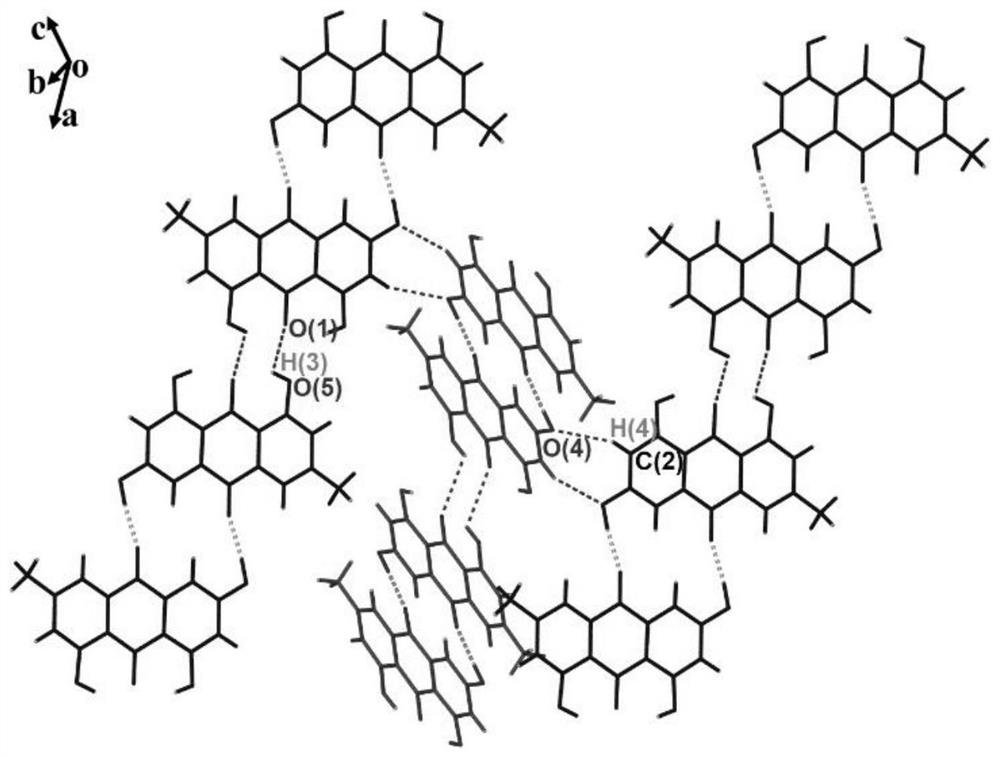
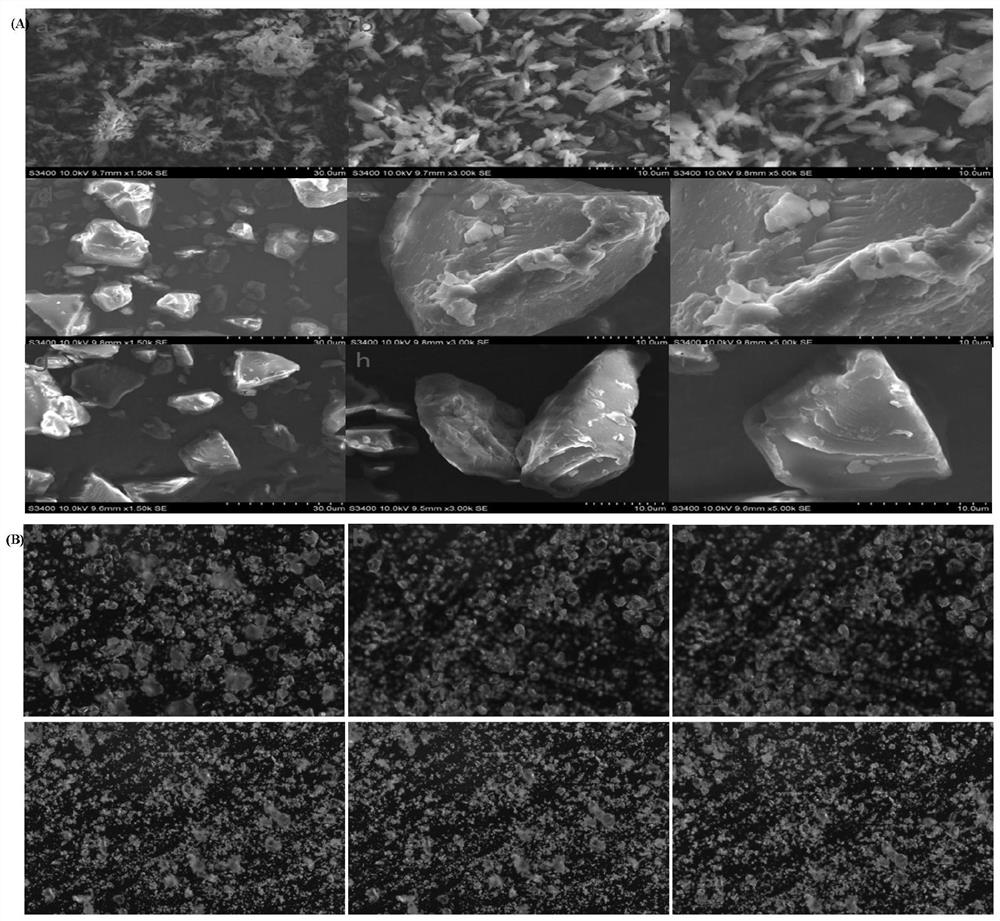
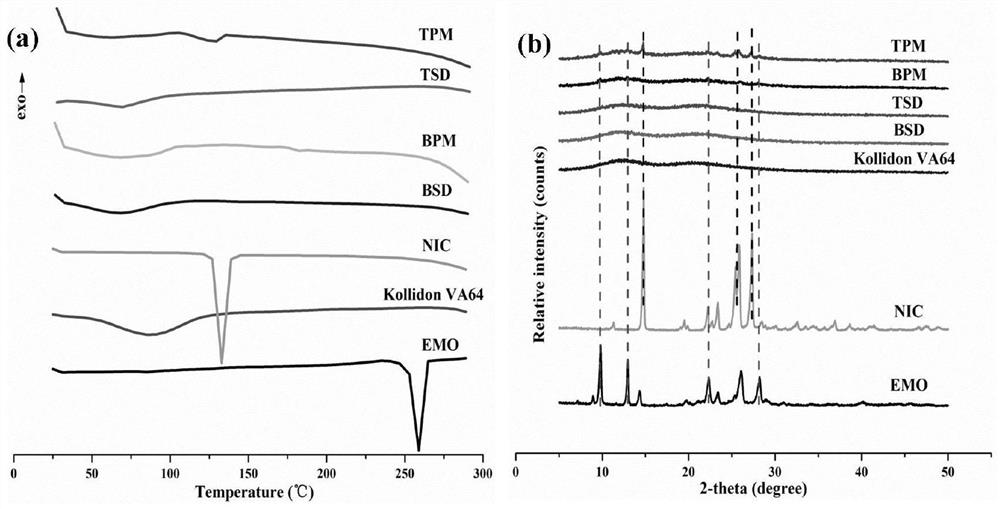
Emodin solid dispersion as well as preparation method and application thereof
A technology of solid dispersion and emodin, applied in the field of medicine, can solve the problems of weakening the advantages of solid dispersion, and achieve the effects of reducing Gibbs free energy and electrical conductivity, enhancing interaction, and improving supersaturation and stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Prescription: Preparation of Emodin Ternary Solid Dispersion by Hot Melt Extrusion (1 / 1 / 6)
[0052]
[0053] Weigh 60g of polyvinylpyrrolidone, 10g of emodin raw material, and 10g of nicotinamide in a polyethylene bag and mix thoroughly for 10min, then transfer to a twin-screw hot-melt extruder, and fully melt at 160°C for 10min at 10rpm. Then gradually increase the rotating speed and place it at 40rpm to extrude; after the extrudate is cooled to room temperature, use a small pulverizer to pulverize it, pass through a 100-mesh pharmacopoeia sieve, and obtain the emodin ternary solid dispersion.
Embodiment 2
[0055] Prescription: Preparation of Emodin Ternary Solid Dispersion by Hot Melt Extrusion (1 / 2 / 5)
[0056]
[0057] Weigh 50g of polyvinylpyrrolidone, 10g of emodin raw material, and 20g of nicotinamide in a polyethylene bag and mix thoroughly for 10 minutes, then transfer to a twin-screw hot-melt extruder, and fully melt at 160°C for 10 minutes at 10 rpm. Then gradually increase the rotating speed and place it at 40rpm to extrude; after the extrudate is cooled to room temperature, use a small pulverizer to pulverize it, pass through a 100-mesh pharmacopoeia sieve, and obtain the emodin ternary solid dispersion.
Embodiment 3
[0059] Prescription: Preparation of Emodin Ternary Solid Dispersion by Hot Melt Extrusion (2 / 1 / 5)
[0060]
[0061] Weigh 60g of polyvinylpyrrolidone, 20g of emodin raw material, and 10g of nicotinamide in a polyethylene bag and mix thoroughly for 10 minutes, then transfer to a twin-screw hot-melt extruder, and fully melt at 160°C for 10 minutes at 10 rpm. Then gradually increase the rotating speed and place it at 40rpm to extrude; after the extrudate is cooled to room temperature, use a small pulverizer to pulverize it, pass through a 100-mesh pharmacopoeia sieve, and obtain the emodin ternary solid dispersion.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com