Method for synthesizing fluorescent functional polyester-based amphiphilic polymer
A functional molecule, polyester-based technology, applied in the field of synthesizing fluorescent functional polyester-based amphiphilic polymers, can solve the problems of difficult polymer end group and topology regulation, poor biocompatibility, uncontrollable polymerization, etc. Excellent biodegradability, easy stability, low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
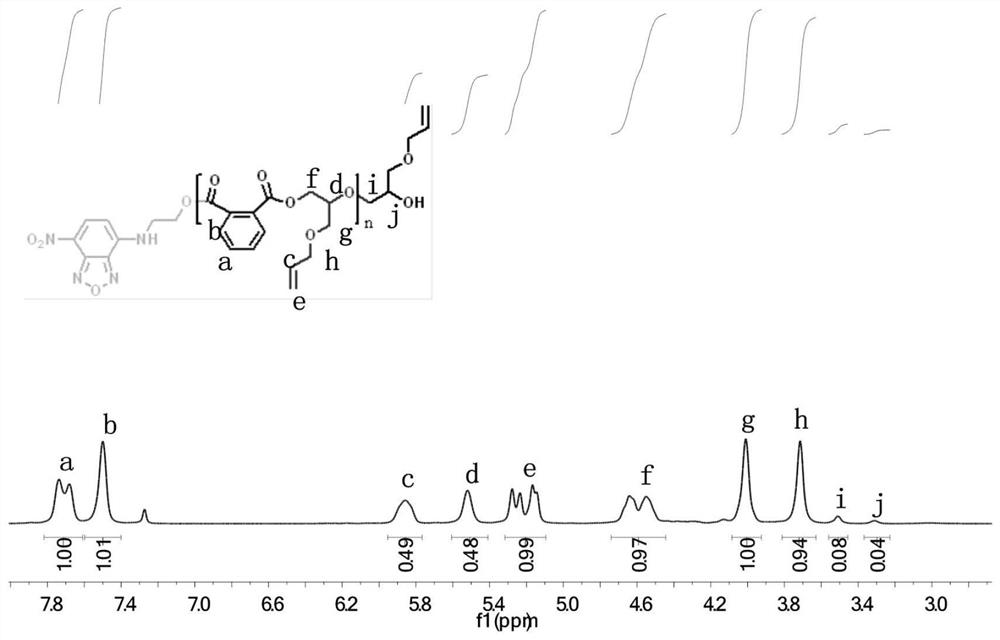
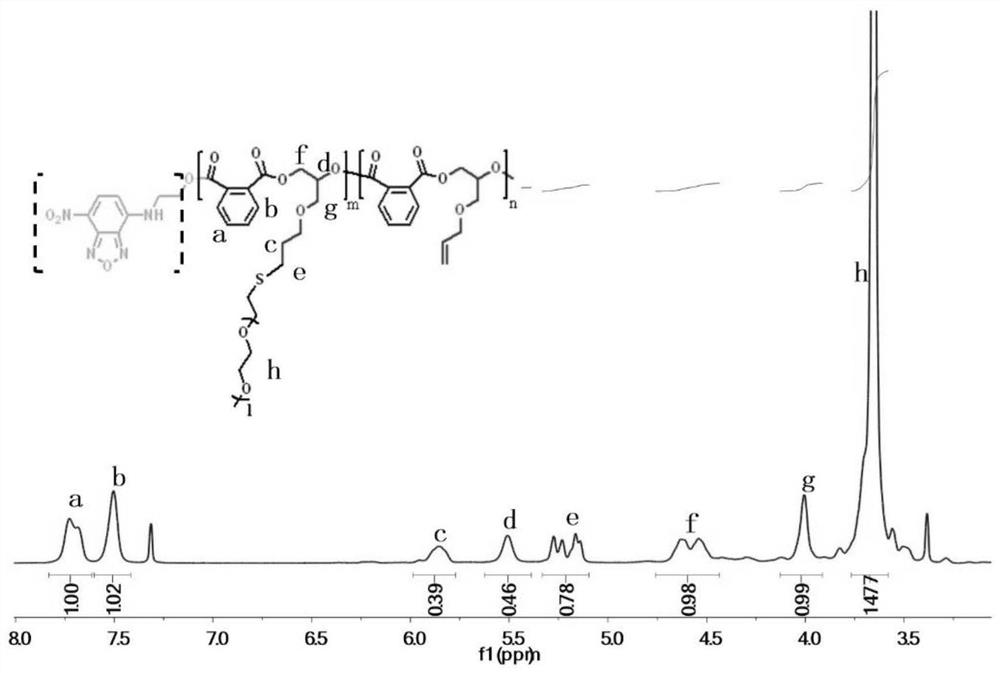
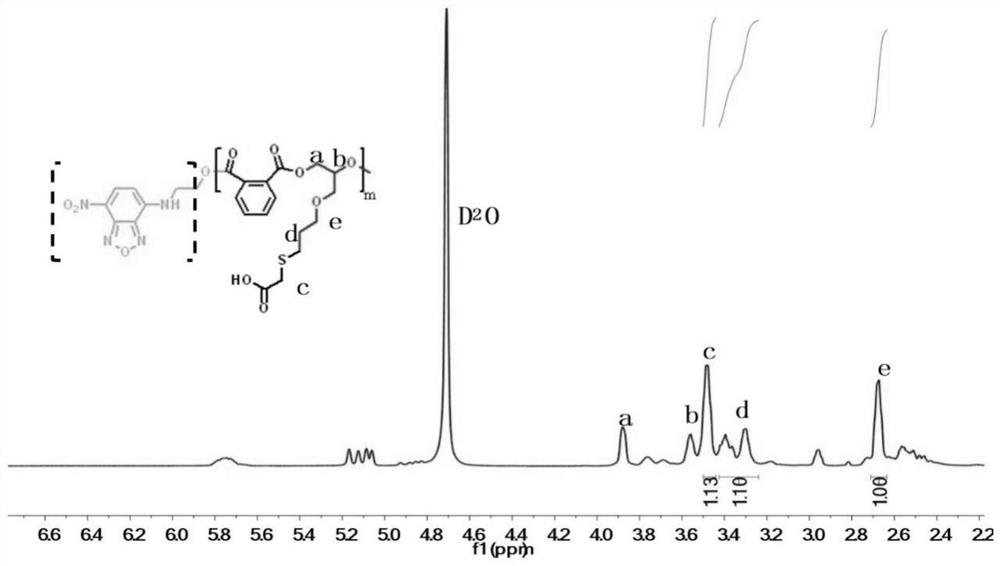
[0042] In a nitrogen-filled glove box, add NBD-OH as an initiator in a dry polymerization reaction tube, and then add monohydroxy functionalized 7-nitrobenzo-2-oxa-1,3-oxadiazole (NBD-OH) (13.9mg, 0.05mmol), allyl glycidyl ether (0.593mL, 0.005mol), phthalic anhydride (sublimed twice) (0.74g, 0.005mol), triethylboron (TEB) / THF solution (20μL, 0.02mmol), phosphazene base (t-BuP 1 ) (5 μL, 0.02 mmol) and THF (2 mL). After feeding, shake evenly to dissolve the monomer, initiator and catalyst completely under stirring. Take it out and place it in an oil bath at 80°C under magnetic stirring for 18 hours. After the reaction, take a small amount of crude product for NMR test to calculate the monomer conversion rate. Subsequently, the crude product was precipitated three times in a mixed solvent of diethyl ether and n-hexane to obtain a purified product, which was placed in a vacuum drying oven to dry to obtain an alternating polyester with a conversion rate of phthalic anhydride mo...
Embodiment 2
[0045] The polymerization conditions are the same as in Example 1, the difference is that hydroxyrhodamine (Rh-OH) is used as the initiator, the other feeding ratios are the same, the acid anhydride is sublimated twice, reacted in an oil bath at 80°C for 18 hours, and the conversion rate of phthalic anhydride monomer 90%, the resulting polyester yield was 64%. The SEC weight average molecular weight of the obtained polymer was 5.8k Da, and the molecular weight distribution was 1.45.
Embodiment 3
[0047] The polymerization conditions were the same as in Example 1, except that 9-anthracene acetic acid was used as the initiator, the other feeding ratios were the same, the acid anhydride was sublimated twice, and the reaction was carried out in an oil bath at 80°C for 18 hours. The conversion rate of phthalic anhydride monomer was 96%, and the obtained The polyester yield was 67%. The SEC weight average molecular weight of the obtained polymer was 6.4k Da, and the molecular weight distribution was 2.23.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com