TiO2-x-based hindered Lewis acid-base pair photocatalyst as well as preparation method and application thereof
A Lewis acid-base and photocatalyst technology, which is applied in the direction of catalyst activation/preparation, physical/chemical process catalysts, chemical instruments and methods, etc., can solve difficult problems such as Lewis acid-base regulation, which is beneficial to adsorption and activation, The effect of mild reaction conditions and wide CO2 content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] Hindered Lewis acid-base pair TiO 2-x The preparation method of material photocatalyst and application thereof, comprises the following steps:
[0036] (1) 1mmol salt (MCl 2 、MCO 3 , M(NO 3 ) 2 , M=Mg, Ca, Sr, Ba, Mn, Fe, Co, Ni, Cu, Zn) were dissolved in 10-40mL of a mixed solution of ethanol and polyethylene glycol, the solution was stirred evenly, and then added to the above solution 1mmol of titanium source (n-butyl titanate, isopropyl titanate), stir evenly, add 6mmol of alkaline mineralization reagent (NaOH, KOH), stir evenly, transfer to a reaction kettle and put it in an oven at 150-200°C for 12 ~25h, the perovskite material MTiO was obtained after centrifugal washing 3 .
[0037] (2) Weigh 0.5mmol of the above-prepared MTiO 3 Add it to the mixed solution of 20-60mL water and polyethylene glycol, then add 1mmol chelating reagent (EDTA-2Na or its hydrate), after the solution is stirred evenly, put it into the reaction kettle and react in an oven at 150-200...
Embodiment 1
[0039] Example 1CaTiO 3 Catalyst preparation
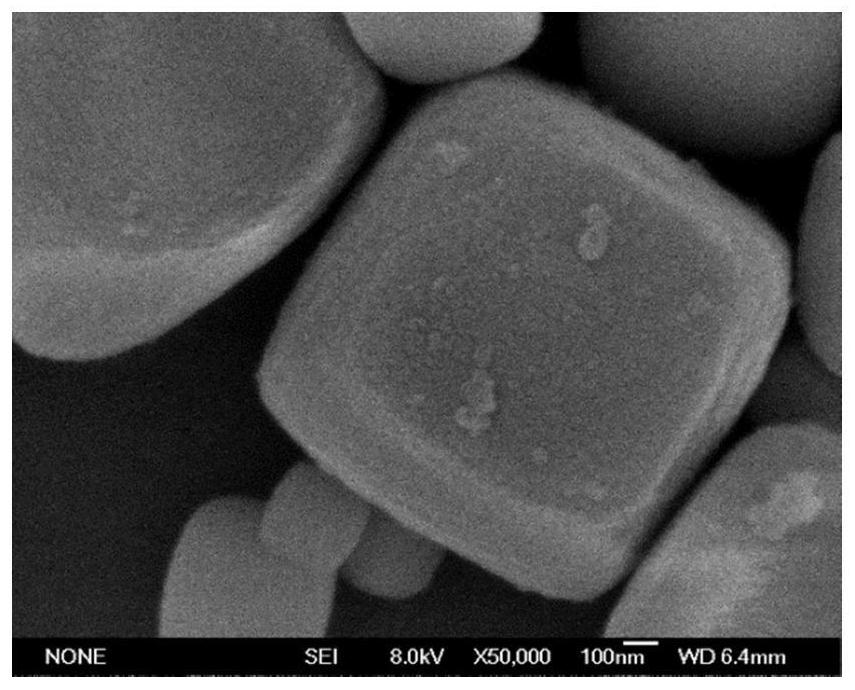
[0040] (1) 1mmol CaCl 2 ·H 2 O was dissolved in 20mL of a mixed solution of ethanol and polyethylene glycol, and the solution was stirred evenly, then 1mmol of titanium source (n-butyl titanate, isopropyl titanate) was added to the above solution, and 6mmol of alkaline Mineralizing reagents (NaOH, KOH), stirred evenly, then transferred to the reaction kettle and placed in an oven at 180°C for 15 hours of reaction. The perovskite material MTiO was obtained after centrifugal washing 3 (Such as figure 1 shown).
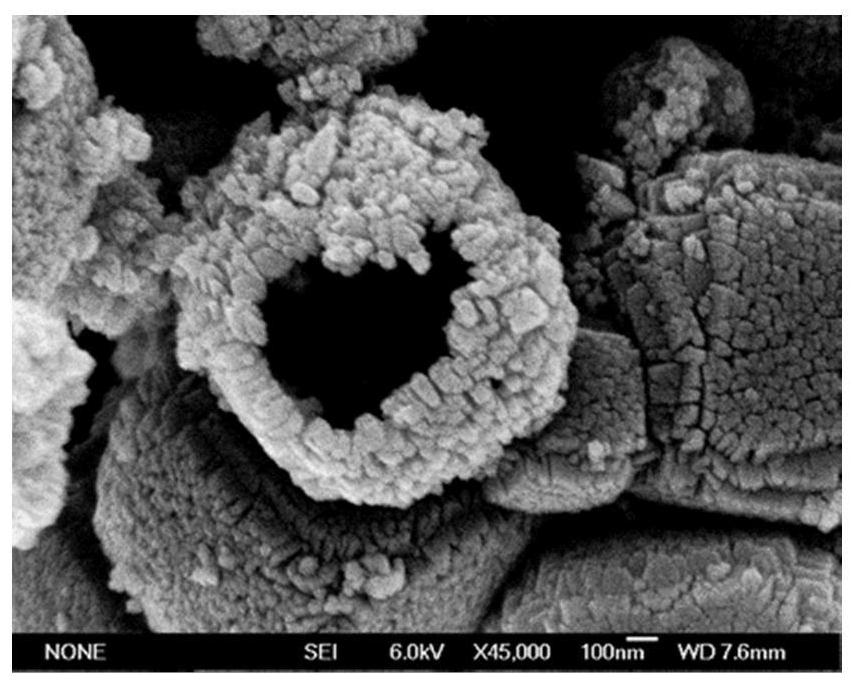
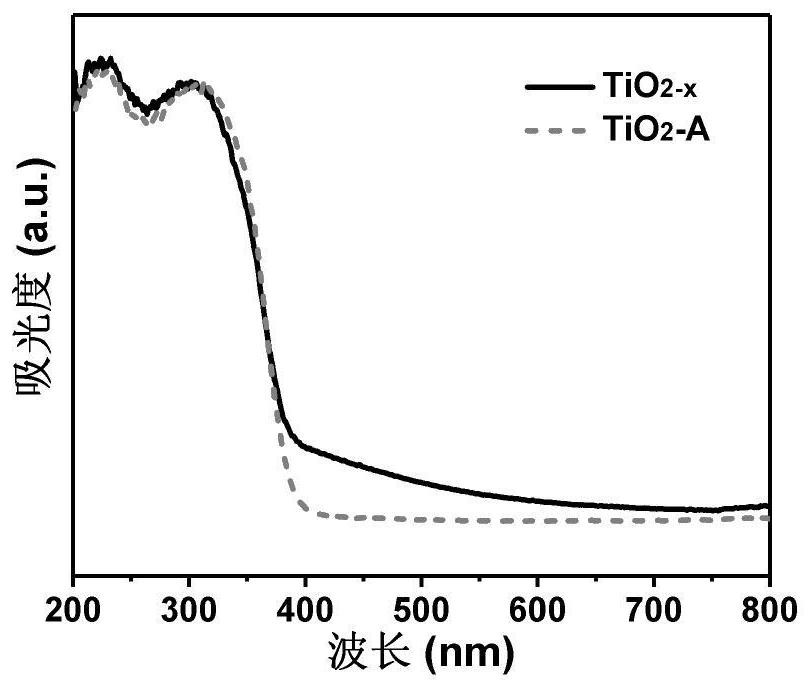
[0041] (2) Weigh 0.5mmol of the above-prepared CaTiO 3 Add it to the mixed solution of 40mL water and polyethylene glycol, then add 1mmol chelating reagent (EDTA-2Na or its hydrate), after the solution is stirred evenly, put it into the reaction kettle and react in an oven at 180°C for 12h, then centrifuge and wash Obtaining TiO Containing Hindered Lewis Acid-Base Pairs 2 Photocatalyst (morphology such as figure 2 ...
Embodiment 2
[0043] Example 2MgCl 2 Catalyst preparation
[0044] (1) 1.2mmol MgCl 2 Dissolve in a mixed solution of 30mL ethanol and polyethylene glycol, stir the solution evenly, then add 1mmol titanium source (n-butyl titanate, isopropyl titanate) to the above solution, stir evenly, add 4mmolNaOH reagent, stir After uniformity, it was transferred to a reaction kettle and placed in an oven at 180°C for 15 hours of reaction. The perovskite material MgTiO was obtained after centrifugal washing 3 .
[0045] (2) Weigh 0.5mmol of the above-prepared MgTiO 3 Add it to the mixed solution of 30mL water and polyethylene glycol, then add 1mmol chelating reagent (EDTA-2Na or its hydrate), after the solution is stirred evenly, put it into the reaction kettle and react in an oven at 180°C for 12h, then centrifuge and wash Obtaining TiO Containing Hindered Lewis Acid-Base Pairs 2 catalyst of light.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com