Application of Lewis acid-base pair in polymerization-induced self-assembly, fiber morphology amphiphilic block polymer as well as preparation method and application of fiber morphology amphiphilic block polymer
A Lewis acid-base pairing and polymerization-inducing technology, which is applied to medical preparations of non-active ingredients, chemical instruments and methods, and pharmaceutical formulations, and can solve problems such as long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] In the present invention, the Lewis base is preferably self-made. In the present invention, the preparation method of the Lewis base preferably includes the following steps: mixing ammonia water, alcohol solvent, propionaldehyde and benzil, and performing a nucleophilic addition reaction to obtain intermediate a; The basic reagent, methyl iodide and a solvent are mixed, and a nucleophilic substitution reaction is carried out to obtain an intermediate b; the intermediate b, KH and a solvent are mixed, and an elimination reaction is carried out to obtain a Lewis base having a structure shown in formula I;
[0044]
[0045] In the present invention, the preparation route of the Lewis base is shown in formula (2):
[0046]
[0047] The invention mixes ammonia water, alcohol solvent, propionaldehyde and benzil, and performs nucleophilic addition reaction to obtain intermediate a. In the present invention, the concentration of the ammonia water is preferably 25-28 wt%,...
Embodiment 1
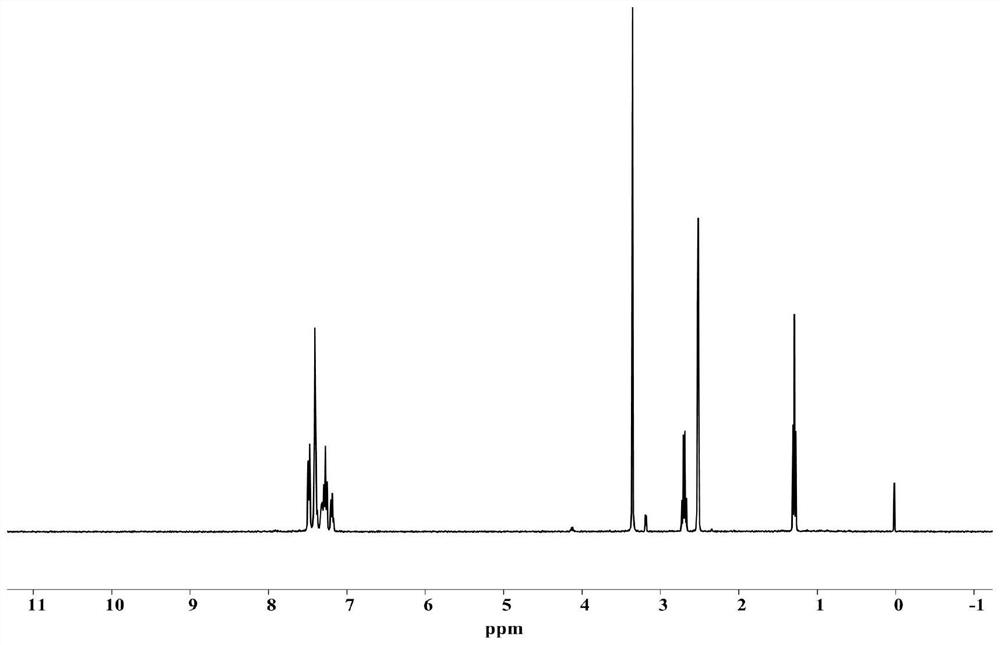
[0065]7.8g (with NH in ammonia 3 Add ammonia water with a concentration of 26wt% into 150mL of methanol and cool to 0°C, add 3.3g of propionaldehyde and 12g of benzil, mix them, and perform nucleophilic addition reaction at 65°C for 12 hours in a sealed environment, filter after concentration, and diethyl ether After washing the solid, it was vacuum-dried to constant weight at 30° C. to obtain intermediate a (8.5 g, yield 60%); the hydrogen spectrum of intermediate a was as follows: figure 1 shown.
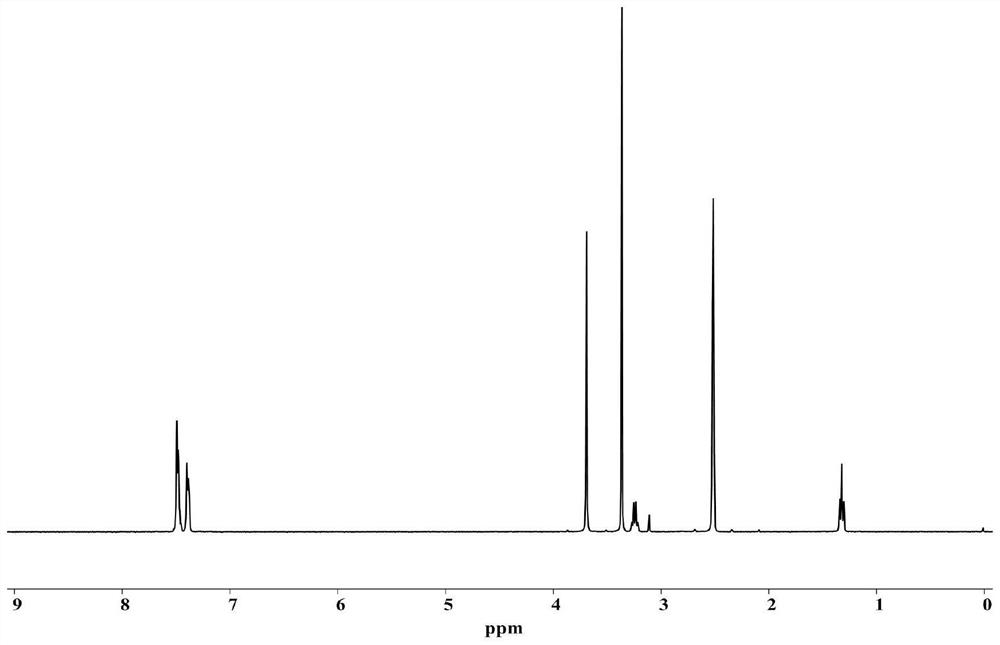
[0066] Mix 7g of intermediate a, 15.5g of potassium carbonate and 150mL of acetonitrile, add 16g of iodomethane, and perform an affinity substitution reaction at 100°C for 24 hours, filter while hot, concentrate the obtained filtrate to half, and filter the obtained solid product with THF Washing, drying in vacuo at 50°C for 12h, to obtain intermediate b (8g, yield 71%); the hydrogen spectrum of intermediate b is as follows: figure 2 shown.
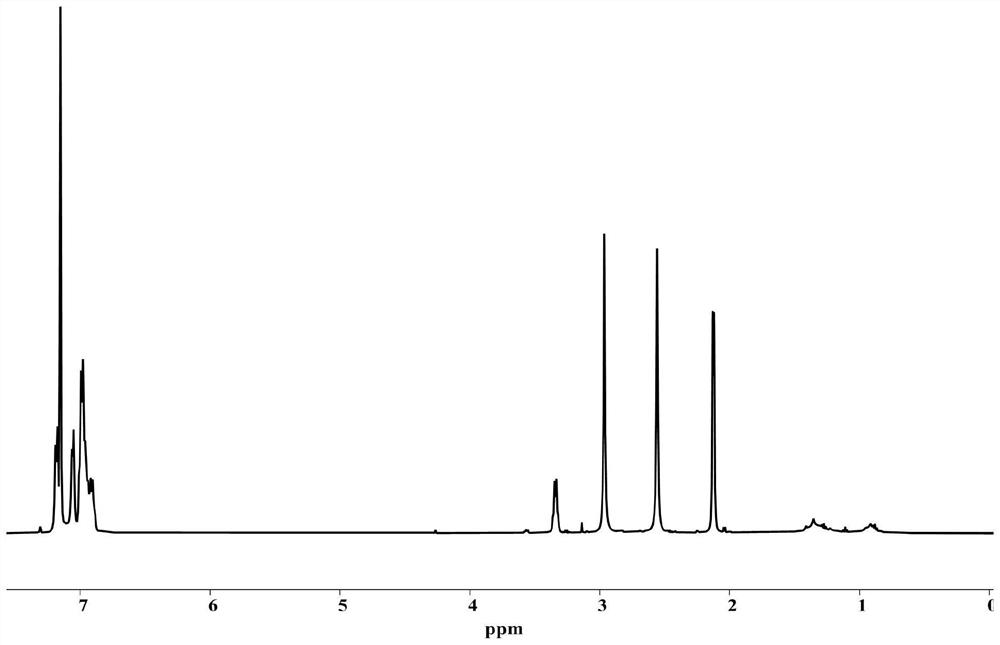
[0067] Add 2 g of intermediate b t...
Embodiment 2
[0069] Dissolve TFEMA in fluorobenzene, add MeAl(BHT) 2 After premixing with the monomer for 2min, add NHO to carry out the first polymerization reaction, by 1 HNMR judged that the TFEMA monomer had completely converted, and the first polymerization reaction time was 1min to obtain a stable segment polymer (PTFEMA 100 , degree of polymerization DP=100); Add HDFDMA monomer to carry out the second polymerization reaction, by 1 HNMR judges that HDFDMA has completely converted, and the second polymerization reaction time is 1min to obtain a fiber-shaped amphiphilic block polymer (abbreviated as PTFEMA 100 -b-PHDFDMA 25 , wherein, 100 represents the polymerization degree DP=100 of PTFEMA, and 25 represents the polymerization degree DP=25 of PHDFDMA), wherein, NHO, MeAl(BHT) 2 , The molar ratio of TFEMA and HDFDMA is 1:2:100:25, and the solid content is 15%.
[0070] Take 0.1mL of the fiber-shaped amphiphilic block polymer solution and dilute it until the concentration of the fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com