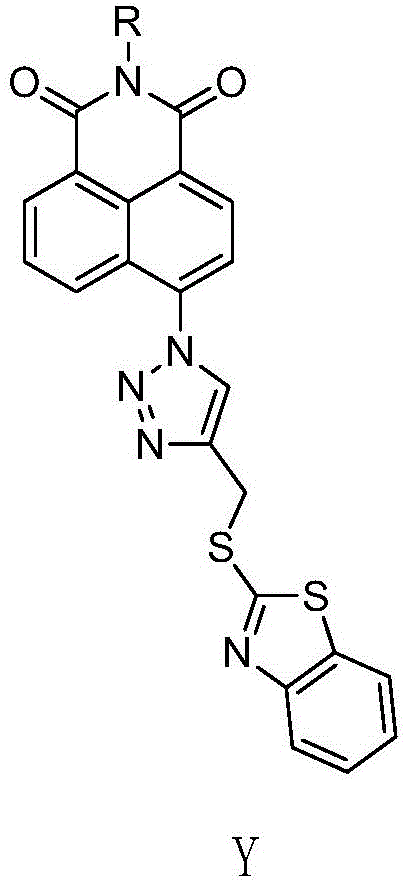
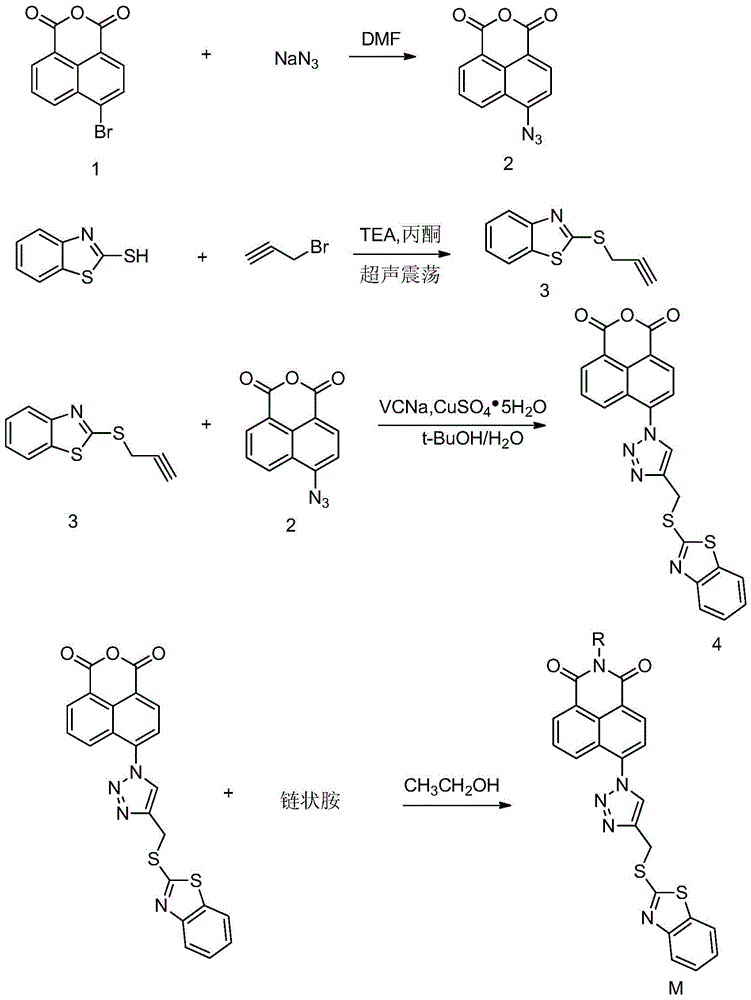
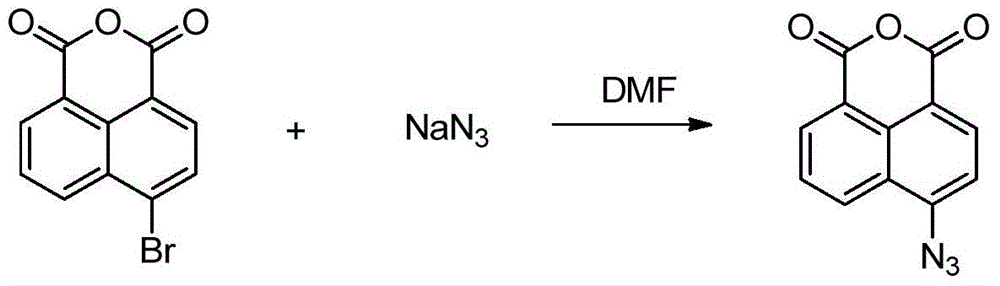A class of naphthalimide compounds containing 2-mercaptobenzothiazole and triazole heterocycle, its preparation method and application
A technology of mercaptobenzothiazole and naphthalimide, applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve problems such as toxic and side effects, achieve the effect of improving biological activity and antitumor performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] N-(N',N'-dimethylaminoethyl)-6-(4-((benzothiazole-2-mercapto)methyl)-[1,2,3]-triazole)naphthoyl Synthesis of imine (compound M1):
[0036] (1) 4-Azide-1.8-naphthalimide (Intermediate 2)
[0037]
[0038] Add 2.5g (9mmol) 4-bromo-1,8 naphthalene anhydride into a 50mL two-necked flask, stir and dissolve with 35mL DMF, slowly add 0.87g (13.5mmol) sodium azide to the reaction system, heat to 55°C, and reflux Magnetically stirred for 1 h, cooled, and the reaction solution was poured into water, a large amount of yellow precipitate was precipitated, filtered and dried to obtain 2.04 g of yellow solid, yield: 95.1%.
[0039] (2) 2-(2-Alkyne-1-propylthio)benzo[d]thiazole (Intermediate 3)
[0040]
[0041] Weigh 4.02g (24mmol) of 2-mercaptobenzothiazole, dissolve it with 40mL of acetone, add 3.34mL (24mmol) of triethylamine, and the reaction solution is an orange transparent liquid, add 1.88mL (24mmol) of propyl bromide alkyne, ultrasonically oscillated for 10 minutes, st...
Embodiment 2
[0051] N-(N',N'-diethylaminoethyl)-6-(4-((benzothiazole-2-mercapto)methyl)-[1,2,3]-triazole)naphthoyl Synthesis of imine (compound M2):
[0052] Except that N,N-diethylethylenediamine was used instead of N,N-dimethylethylenediamine in (4), other synthesis and experimental treatment methods were the same as in Example 1. Separation by silica gel column chromatography (column chromatography eluent is CH 2 Cl 2 :CH 3 OH=12:1), the target product M2 was obtained as light brown solid with a yield of 70.6%. Melting point: 114.3-115.9°C.
[0053] 1 H NMR (400MHz, CDCl 3 )δ8.66(dd, J=7.4,4.1Hz,2H),8.16(s,1H),8.11(d,J=8.6Hz,1H),7.88(d,J=8.1Hz,1H),7.79( t,J=7.8Hz,2H),7.69(t,J=7.9Hz,1H),7.43(t,J=7.6Hz,1H),7.33(t,J=7.6Hz,1H),4.85(s, 2H), 4.35(t, J=6.6Hz, 2H), 2.90(s, 2H), 2.77(s, 4H), 1.14(s, 6H).
[0054] +ESI MS(M+H):C 28 h 26 N 6 o 2 S 2 , calculated value: 543.1559, measured value: 543.1635.
Embodiment 3
[0056] N-(N',N'-dimethylaminopropyl)-6-(4-((benzothiazole-2-mercapto)methyl)-[1,2,3]-triazole)naphthoyl Synthesis of imine (compound M3):
[0057] Except that N,N-dimethylpropylenediamine was used instead of N,N-dimethylethylenediamine in (4), other synthesis and experimental treatment methods were the same as in Example 1. Separation by silica gel column chromatography (column chromatography eluent is CH 2 Cl 2 :CH 3 OH=12:1), the target product M3 was obtained as light brown solid with a yield of 80.3%. Melting point: 116.0-118.2°C.
[0058] 1 H NMR (400MHz, CDCl 3 )δ8.66(dd, J=7.4,4.7Hz,2H),8.16(s,1H),8.12(d,J=8.5Hz,1H),7.88(d,J=8.1Hz,1H),7.80( t,J=8.5Hz,2H),7.74–7.62(m,1H),7.43(t,J=7.6Hz,1H),7.33(t,J=7.6Hz,1H),4.86(s,2H), 4.27(t, J=6.9Hz, 2H), 2.65(s, 2H), 2.42(s, 6H), 2.05(s, 2H).
[0059] +ESI MS(M+H):C 27 h 24 N 6 o 2 S 2 , calculated value: 529.1402, measured value: 529.1462.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com