Silicon-containing heterocyclic compound and organic electroluminescent element containing same
A compound and silicon heterocycle technology, applied in the field of organic electroluminescence, can solve the problems of reducing the quantum yield of the blue light system, aggravating the quenching of molecular fluorescence, and low quantum efficiency of luminescence, so as to improve the internal quantum efficiency and increase the electron cloud Effect of density, low sublimation temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
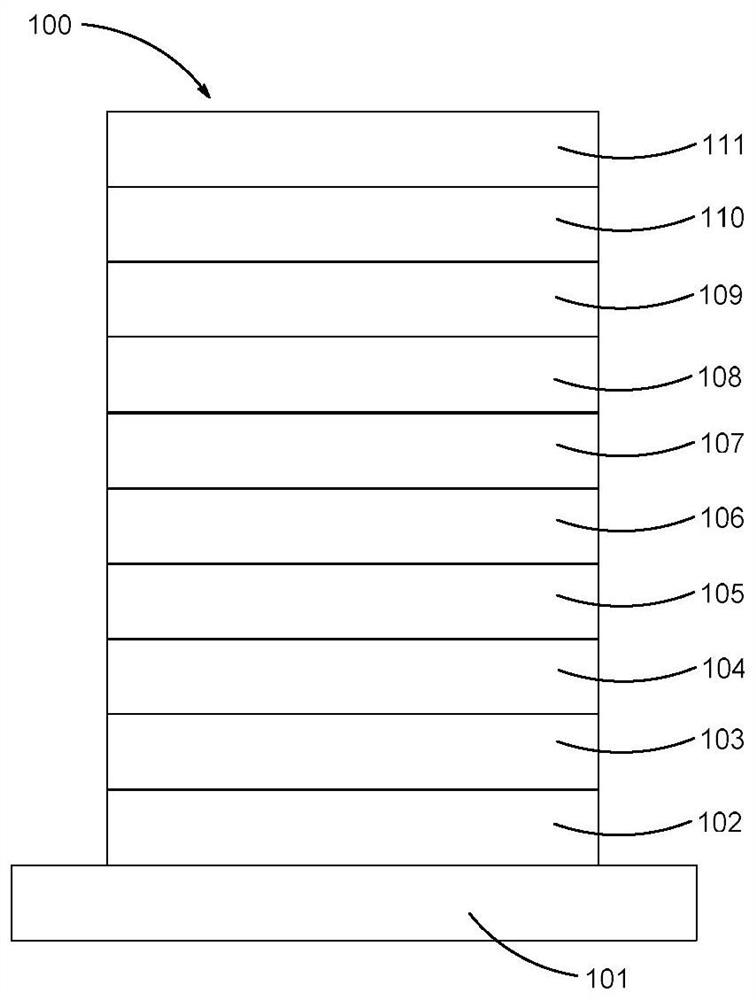
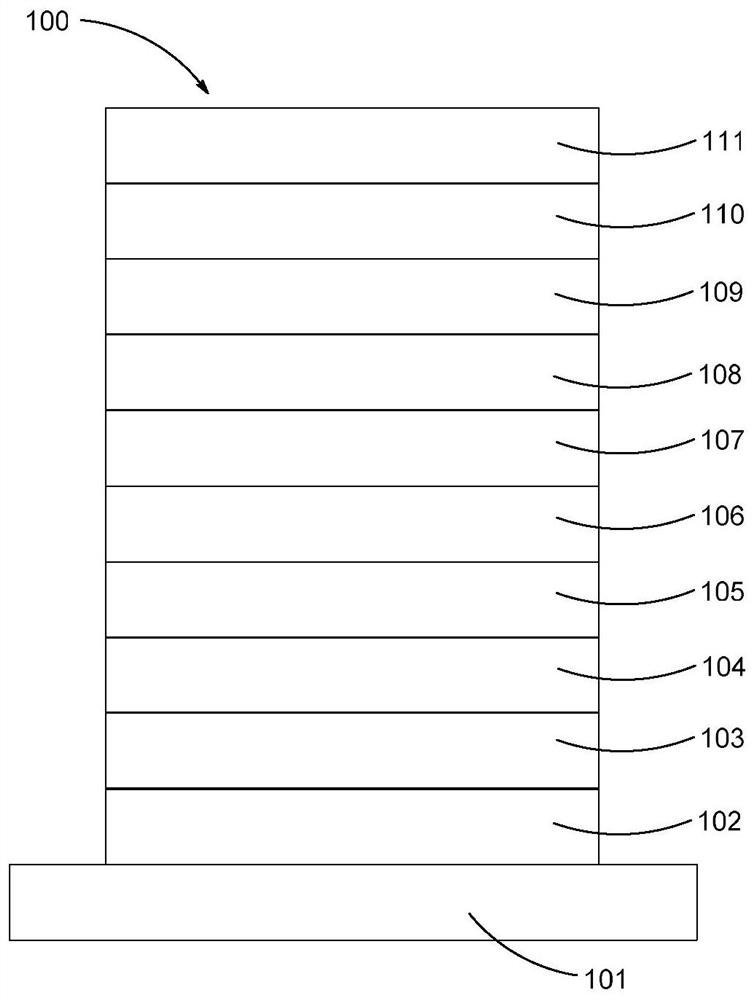
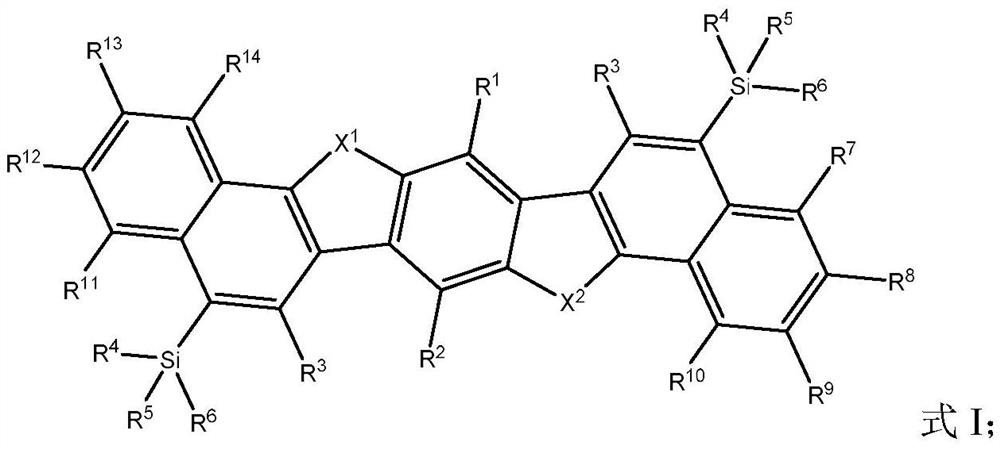
Image
Examples
Embodiment 1
[0086] The preparation method of compound A1, comprises the steps:
[0087] Step 1: Preparation of Compound Int.-1
[0088]
[0089] Under nitrogen protection, 75.0mmol of 1,4-diiodo-2,5-dimethoxybenzene (CAS: 51560-21-5) was dissolved in 60mL of THF and 30mL of triethylamine, and 150.0mmol of p-methoxyphenylacetylene, 7.5mmol of cuprous iodide, 0.75mmol of PdCl 2 (PPh 3 ) 2 Catalyst, stirred and reacted for 12 hours, after the reaction was completed, filtered, the filtrate was concentrated to dryness under reduced pressure, separated and purified by silica gel column, and Int.-1 was obtained as a yellow solid with a yield of 96%.
[0090] The second step: the preparation of compound Int.-2
[0091]
[0092] 50.0 mmol of Int.-1 prepared in the first step was dissolved in 100 mL of dry dichloromethane, and under nitrogen protection, a solution of 0.1 mol of iodine dissolved in dichloromethane was added dropwise, stirred for 3 hours, and 50 mL of 10% Sodium thiosulfat...
Embodiment 2
[0112] The preparation method of compound A2, comprises the steps:
[0113] The first step: preparation of compound Int.-7
[0114]
[0115] The Pd(PPh 3 ) 4 Mix, then add 60mL of THF and 20mL of water, stir and raise the temperature to reflux for 12 hours, cool to room temperature, add 20mL of ethanol, filter, wash the filter cake with water and ethanol, separate and purify with silica gel column to obtain the intermediate Int. -7, yield 82%.
[0116] The second step: the preparation of compound Int.-8
[0117]
[0118] Referring to the synthesis method in the sixth step of Example 1, only Int.-5 in the sixth step of Example 1 was replaced with Int.-7 to prepare Int.-8 with a yield of 84%.
[0119] The third step: the preparation of compound A2
[0120]
[0121] Referring to the preparation method in the seventh step of Example 1, only the intermediate Int.-6 in the seventh step of Example 1 was replaced with the intermediate Int.-8 prepared in the previous step...
Embodiment 3
[0125] The preparation method of compound A11, comprises the steps:
[0126] Step 1: Preparation of Compound Int.-9
[0127]
[0128] 20.0mmol of Int.-4 prepared in Example 1 was dissolved in 40.0mL of dry THF, cooled to -78°C with liquid nitrogen, then 17.6mL of 2.5M n-butyllithium n-hexane solution was added dropwise, and the reaction was stirred for 1 hour , dropwise added 48.0mmol of trimethylchlorosilane, stirred for 1 hour, raised to room temperature, added 20mL of saturated ammonium chloride aqueous solution, extracted with ethyl acetate, collected the organic phase, dried, filtered, and the filtrate was concentrated to dryness under reduced pressure. Separation and purification with a silica gel column gave the intermediate Int.-9 with a yield of 90%.
[0129] The second step: the preparation of compound Int.-10
[0130]
[0131] Referring to the synthesis method in the sixth step of Example 1, only Int.-5 in the sixth step of Example 1 was replaced with Int.-9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com