Application of flavonoid
A kind of technology of flavonoids, hydroxyflavonoid dihydrate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
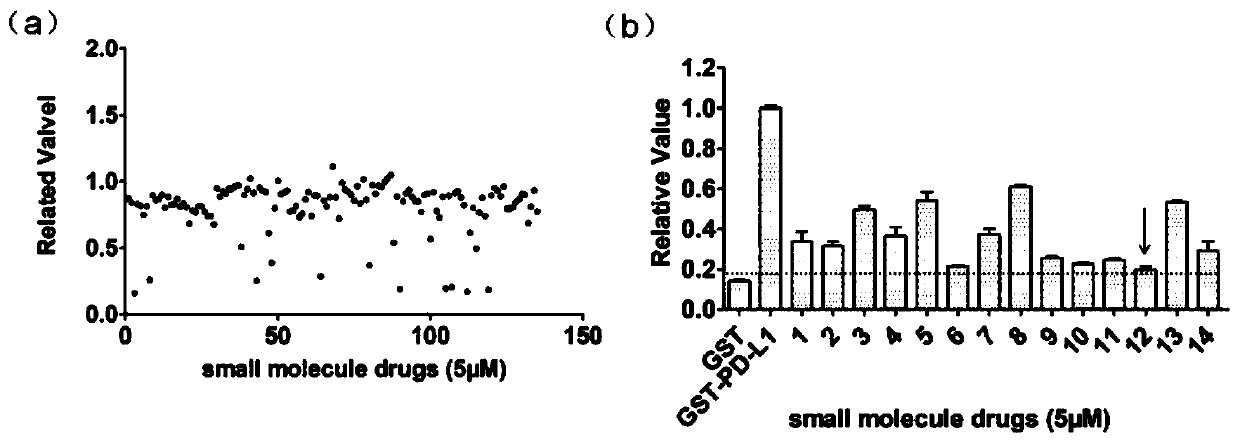
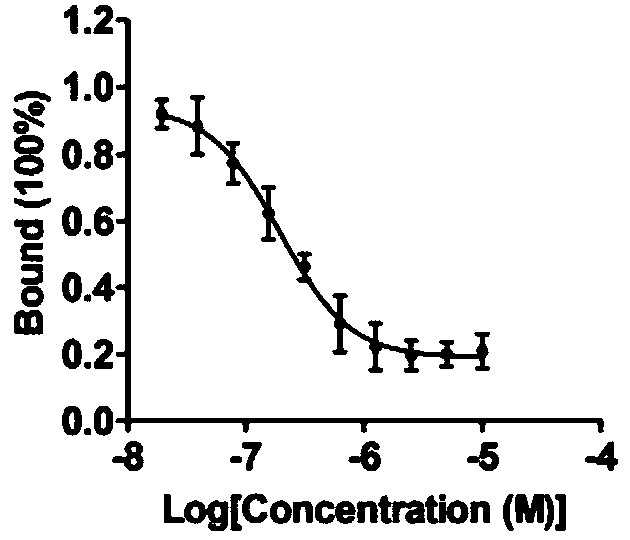
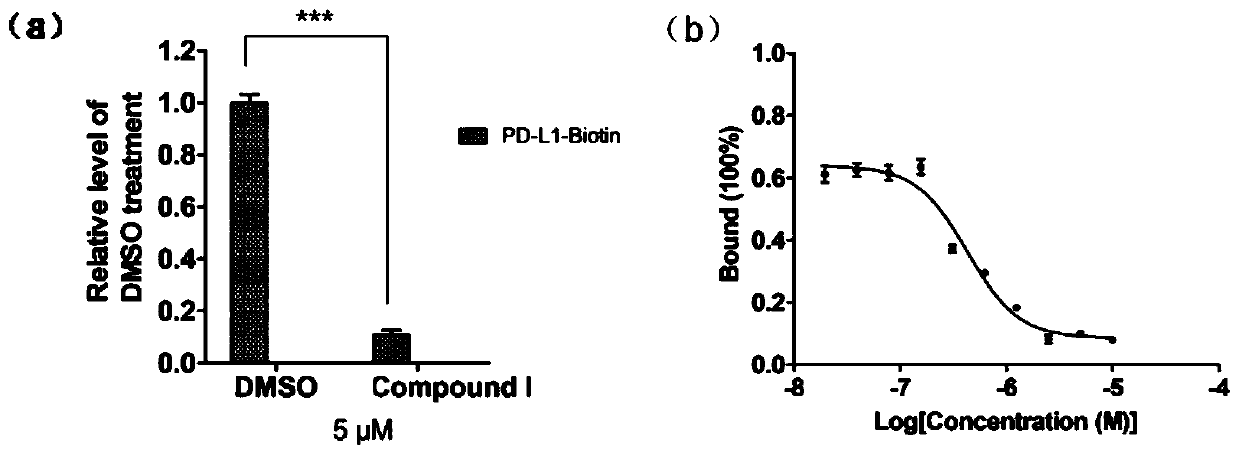
[0029] (1) Screening of inhibitors of PD-1 / PD-L1 protein interaction
[0030] ① Dilute the His-Trx-PD-1 fusion protein expressed by E. coli to 1 μg / mL with carbonate coating buffer, add 100 μL per well to a 96-well microtiter plate, and place at 4 °C overnight. Wash 3 times with 1×PBST, block with 5% skimmed milk powder, 300 μL per well, block at 37°C for 2hrs.
[0031] ② Wash 3 times with 1×PBST. At the same time, dilute GST and GST-PD-L1 proteins to 2 μg / mL with 1×PBS, add 0.2 μL of DMSO or small molecule compound (final concentration 5 μM), mix well, add 100 μL to each well of a 96-well plate, and combine at 37 °C 2hrs. Wash 3 times with 1×PBST, add 100 μL of GST antibody (rabbit polyclonal antibody) diluted in 1×PBS to each well, and bind at 37°C for 2hrs. Wash 3 times with 1×PBST, add 100 μL of HRP-labeled goat anti-rabbit antibody diluted in 1×PBS to each well, bind at 37°C for 0.5hrs.
[0032] ③Wash 5 times with 1×PBST, add 100 μL TMB substrate chromogenic solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com