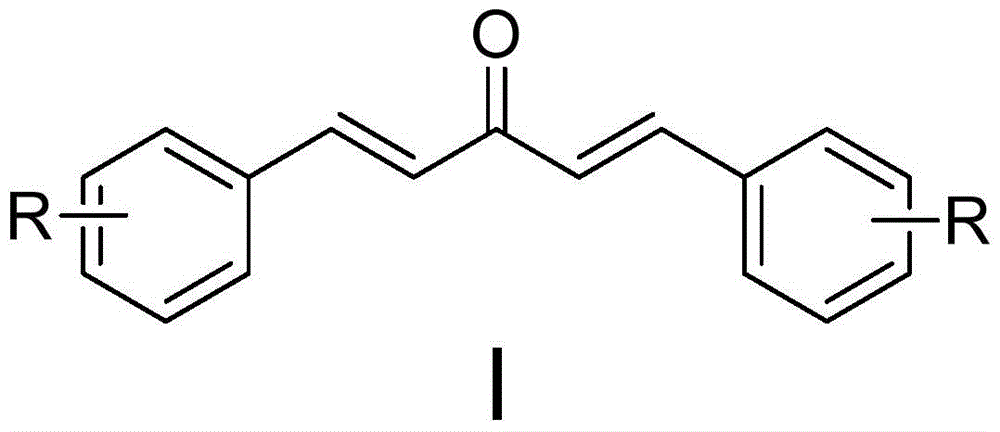
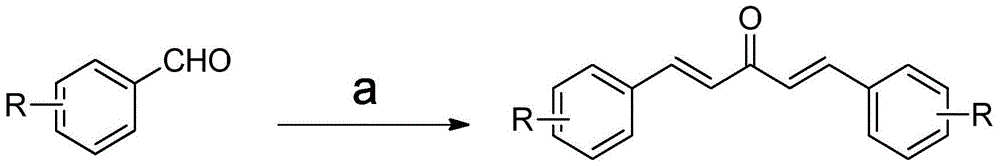
Monocarbonyl curcumin analogs, and preparation method and application thereof
The technology of monocarbonyl curcumin and carbonyl curcumin, which is applied in the field of medicine, can solve the problems of lack of research on the apoptosis induction mechanism of NCI-H460 cells, and achieve the effects of mild reaction conditions, simple preparation process and simple product molecular structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
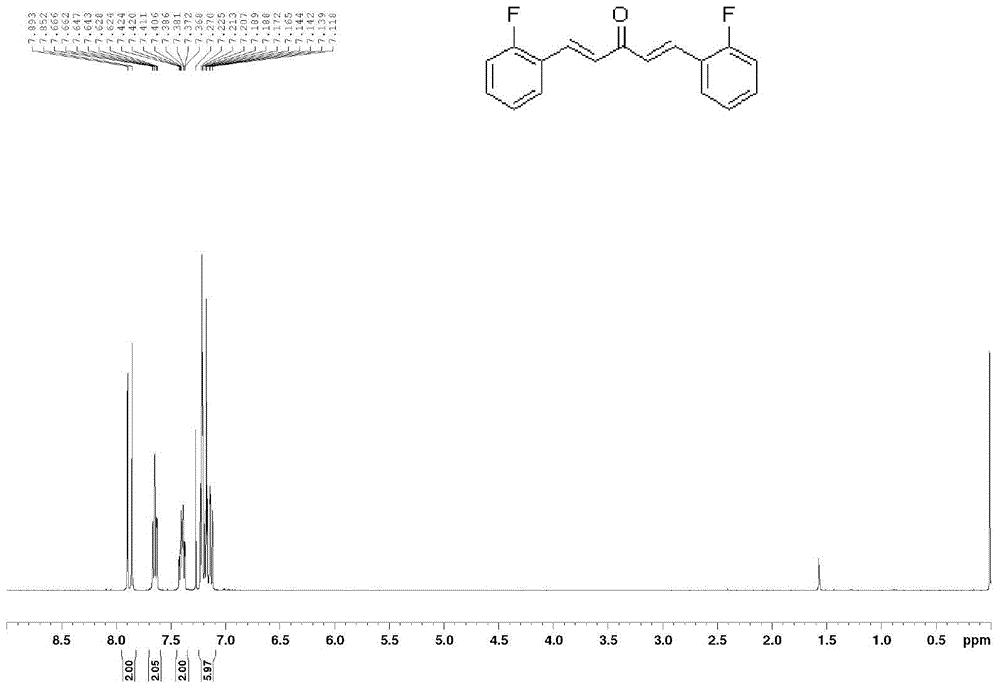
[0048] Preparation of 2,2'-fluoro monocarbonyl curcumin analog (A)
[0049]
[0050] 2-Fluorobenzaldehyde (2.48g, 20mmol) was dissolved in 8mL of absolute ethanol, acetone (0.74mL, 10mmol) was added, and 6mL of 20% sodium hydroxide was slowly added dropwise at -10°C, 0°C, and 10°C respectively After solution, stir at room temperature for 48 hours, add dilute hydrochloric acid to adjust the reaction system to pH = 5, filter, wash with water, and separate by n-hexane / ethyl acetate (15:1) column chromatography to obtain 2,2'-F-monocarbonyl curcumin , and the yields were 70%, 75%, and 68%, respectively. 1 HNMR (400MHz, CD 3 Cl), δ7.85(d, J=16.4Hz, 2H), 7.62(dt, J=7.6, 1.6Hz, 2H), 7.37-7.42(m, 2H), 7.12-7.23(m, 6H); 13 CNMR (100MHz, CD 3 Cl), δ189.0, 162.9, 160.4, 136.1, 131.9, 129.4, 127.6, 124.5, 122.9, 116.4.
Embodiment 2
[0052] Preparation of 3,3'-fluoro monocarbonyl curcumin analog (B)
[0053]
[0054] 3-Fluorobenzaldehyde (2.48g, 20mmol) was dissolved in 8mL of absolute ethanol, acetone (0.74mL, 10mmol) was added, and 6mL of 20% sodium hydroxide was slowly added dropwise at -10°C, 0°C, and 10°C respectively After solution, stir at room temperature for 48 hours, add dilute hydrochloric acid to adjust the reaction system to pH = 5, filter, wash with water, and separate by n-hexane / ethyl acetate (15:1) column chromatography to obtain 2,2'-F-monocarbonyl curcumin , and the yields were 50%, 62%, and 53%, respectively. 1 HNMR400MHz (CD 3 Cl), δ7.68(d, J=16.0Hz, 2H), 7.60-7.63(m, 4H), 7.10(t, J=7.6Hz, 4H), 6.98(d, J=16.0Hz, 2H); 13 HNMR100MHz (CD 3 Cl), δ188.4, 165.3, 162.8, 142.1, 131.0, 130.3, 125.1, 116.3, 116.0.
Embodiment 3
[0056] Preparation of 4,4'-fluoromonocarbonyl curcumin analog (C)
[0057]
[0058] 4-Fluorobenzaldehyde (2.48g, 20mmol) was dissolved in 8mL of absolute ethanol, acetone (0.74mL, 10mmol) was added, and 6mL of 20% sodium hydroxide was slowly added dropwise at -10°C, 0°C, and 10°C respectively After solution, stir at room temperature for 48 hours, add dilute hydrochloric acid to adjust the reaction system to pH = 5, filter, wash with water, and separate by n-hexane / ethyl acetate (15:1) column chromatography to obtain 2,2'-F-monocarbonyl curcumin , and the yields were 60%, 71%, and 59%, respectively. 1 HNMR400MHz (CD 3 Cl), δ7.69(d, J=16.0Hz, 2H), 7.55(d, J=8.7Hz, 4H), 6.89(d, J=8.7Hz, 4H), 6.64(d, J=15.9Hz, 2H), 5.98(s, 1H); 13 HNMR100MHz (CD 3 Cl), δ184.6, 160.6, 141.1, 131.0, 127.7, 122.1, 116.9, 101.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com