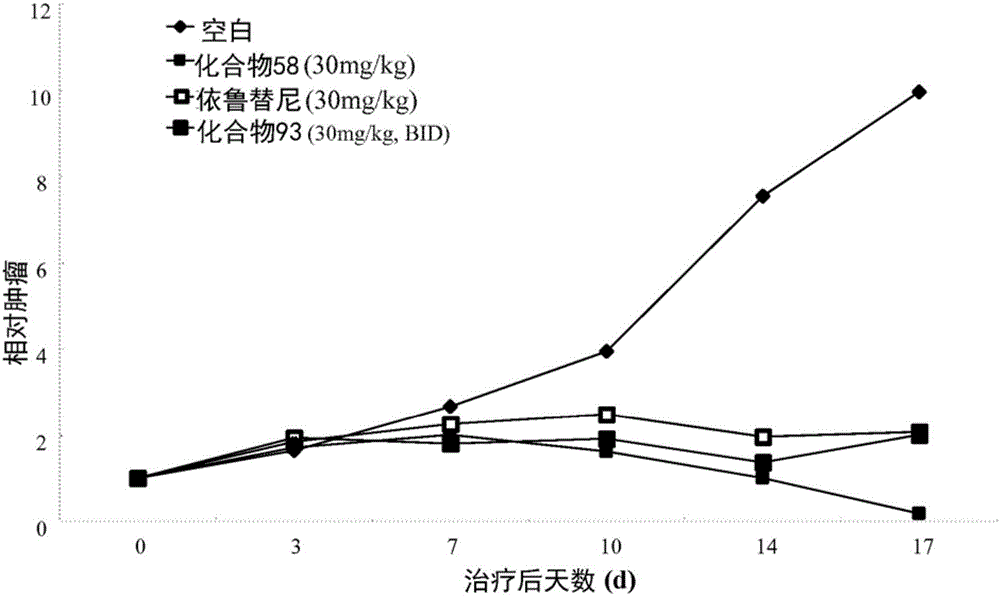
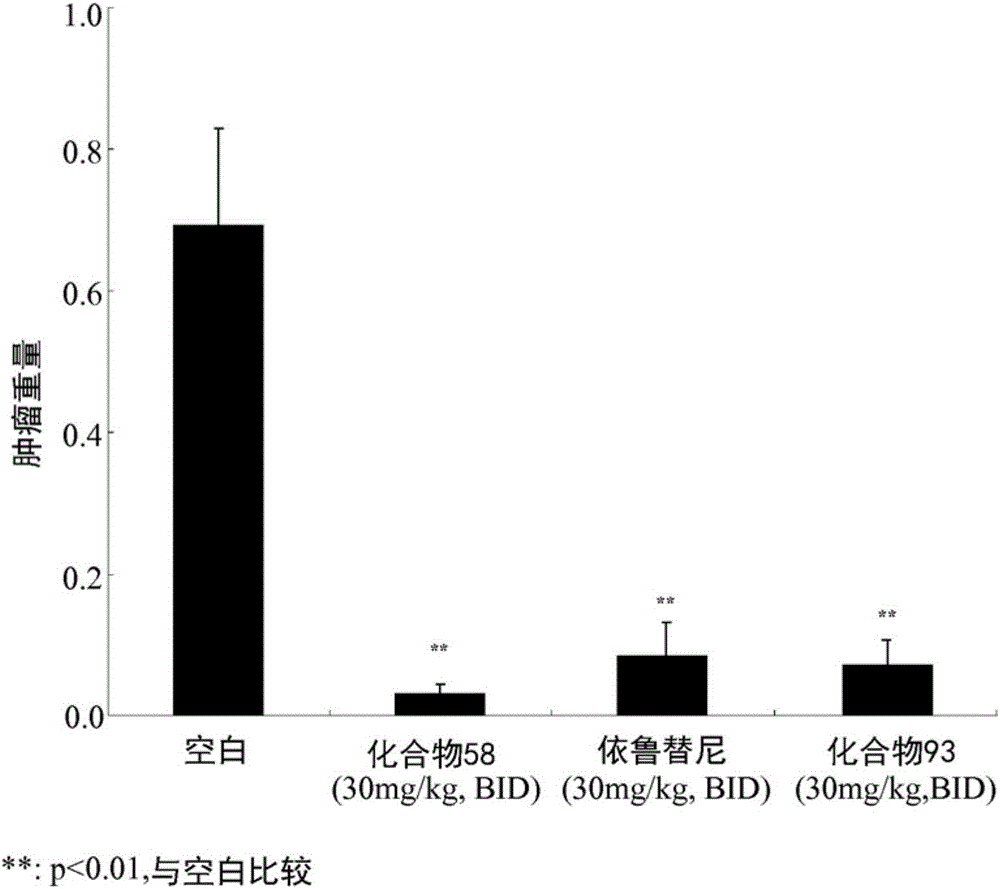
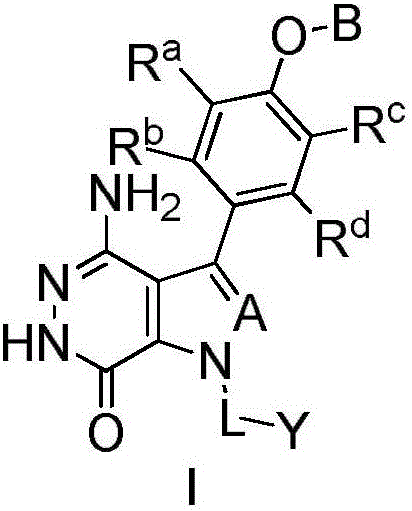
Aminopyridazinone compounds as protein kinase inhibitors
A technology of compounds and solvates, applied in the field of aminopyridazinone compounds as protein kinase inhibitors, can solve problems such as immaturity of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0158] Example 1. (R)-1-(1-acryloylpiperidin-3-yl)-4-amino-3-(4-phenoxyphenyl)-1H-pyrrolo[2,3-d] Pyridazin-7(6H)-one
[0159]
[0160] Step 1A
[0161] To a solution of EtONa (160ml, 21% in EtOH, 0.49mmol) in EtOH (110ml) in an ice bath was added diethyl oxalate (64ml, 0.47mol). The mixture was stirred for 30 min. A solution of 1a (16 g, 0.15 mmol) in EtOH (30 ml) was added. The resulting mixture was stirred overnight at room temperature. After cooling in an ice bath, the suspension was filtered. The solid was washed with a little EtOH, then dissolved in water (380ml). The solution was acidified to pH~4 with HCl. A large amount of solid appeared which was filtered, washed with water and dried to give 1b (11.9g) as a yellow solid.
[0162] Step 1B
[0163]To a solution of 1b (2.3g, 7.5mmol) in EtOAc (120ml) at 60°C was added dropwise a solution of 1c (2.3g, 11.4mmol) in EtOAc (32ml). The mixture was refluxed for 4h. After cooling to room temperature, the solvent wa...
Embodiment 13
[0179] Example 13.1-(1-acryloyl-4-fluoropiperidin-3-yl)-4-amino-3-(4-phenoxyphenyl)-1H-pyrrolo[2,3-d]pyridazine -7(6H)-one
[0180]
[0181] Step 13A
[0182] 13a (2.2g) and NH 4 A mixture of OH (14 mL) in EtOH (33 mL) was stirred at 80 °C for 18 h in a sealed tube. The solvent was removed; the residue was dissolved in THF (30 mL) and EtOH (30 mL) and filled with Boc 2 O (2.46g). The mixture was stirred at room temperature for 20 h. The crude product was purified by silica gel chromatography to give white solid 13b (1.02g) and the undesired regioisomer (1.7g).
[0183] Step 13B
[0184] DAST (0.28 mL, 2.14 mmol) was added dropwise to a solution of 13b (0.68 g, 1.94 mmol) in DCM (20 mL) at -78 °C. The mixture was allowed to warm to room temperature overnight. NaHCO 3 The saturated solution was quenched and extracted with DCM. The organic extract was treated with Na 2 SO 4 Dry, filter and concentrate. The residue was purified by silica gel chromatography to give ...
Embodiment 14
[0199] Example 14. (R)-1-(1-acryloyl-5,5-difluoropiperidin-3-yl)-4-amino-3-(4-phenoxyphenyl)-1H-pyrrolo [2,3-d]pyridazin-7(6H)-one
[0200]
[0201] Step 14A
[0202] To a solution of 1b (271 mg) in EtOAc (12 ml) at 60 °C was added dropwise 14a (223 mg, the compound was prepared according to the procedure described by Anne Cochi et al. in Organic Letters, 2011, vol. 13, p. 4442-4445) in EtOAc (2ml) solution. The mixture was refluxed for 18h. After cooling to room temperature, the solvent was removed. The residue was purified by silica gel chromatography to afford 14b (76 mg) as a pale yellow oil.
[0203] Step 14B
[0204] Combine 14b (65mg) and Pd(OH) 2 / C (10wt%, 50mg) in the mixture of MeOH / THF (3 / 1ml) in H 2 Stir under a balloon for 20 h. The reaction mixture was filtered through a pad of celite, washed with EtOAc / MeOH, and concentrated. The residue was purified by silica gel chromatography to afford 14c (38 mg) as a white solid.
[0205] Step 14C
[0206] To...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com