Preparation method of rgd and peg co-modified pamam dendrimer loaded with arsenic trioxide drug delivery system
A technology of arsenic trioxide and dendrimers, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve the lack of specificity of distribution in the body, intracranial central tissue adverse reactions, ATO Poor fat solubility and other problems to achieve the effect of improving pharmacokinetic characteristics, increasing bioavailability, and reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
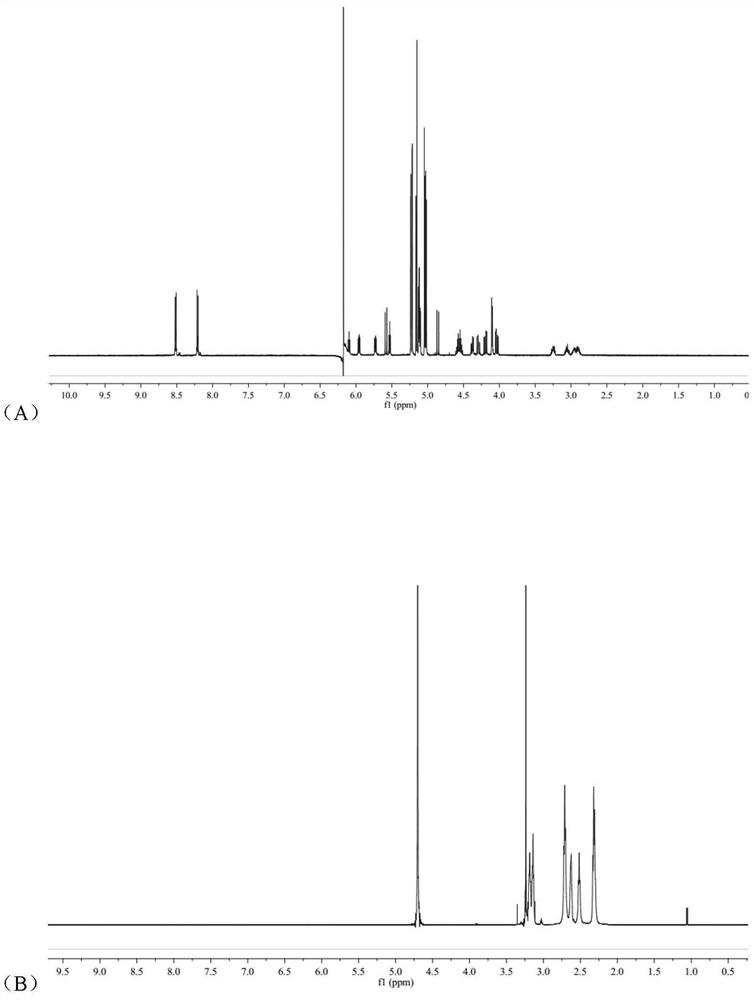
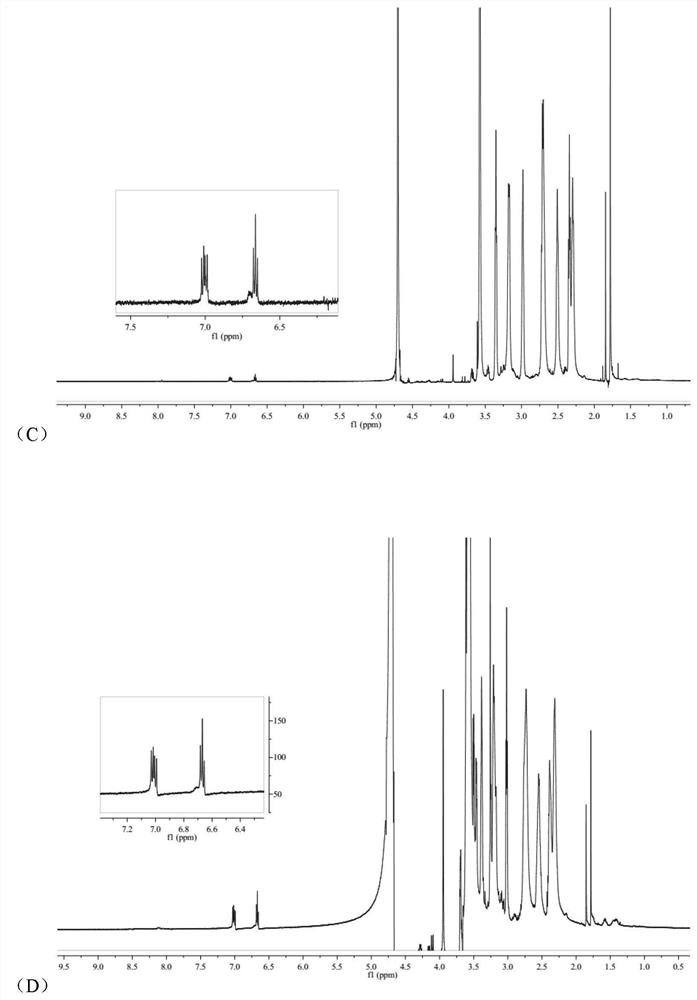
Image
Examples
Embodiment 1
[0043] (1) Preparation of arsenic solution:
[0044] Weigh a certain amount of arsenic trioxide, add concentrated hydrochloric acid drop by drop to it until it is completely dissolved, then adjust the pH to 7.4 with concentrated sodium hydroxide solution to make arsenic salt mother liquor with a concentration of about 1 mg / mL.
[0045] (2) Preparation of RGDyC-PEG-PAMAM:
[0046] Weigh 4.68mg RGDyC, dissolve in 2mL acetic acid-sodium acetate buffer (pH 6.0), add 27.32mgMAL-PEG to it 3500 -NHS, vortexed for 30s, quickly added to 2 mL of borax-sodium hydroxide buffer (pH 9.2) containing 5.0 mg of PAMAM G5, and reacted overnight at room temperature. The pH of the system was adjusted to 7.0 by buffer, and 5 μL of β-mercaptoethanol was added to react for 1 h to terminate the unreacted maleimide groups. Use a Millipore ultrafiltration centrifuge tube with a cut-off molecular weight of 10,000 to perform ultrapure water ultrafiltration and centrifugation, and freeze-dry to obtain 15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com