A kind of non-viral gene carrier for gene delivery and its preparation method and application
A gene carrier and non-viral technology, applied in the field of non-viral gene carrier for gene delivery and its preparation and application, can solve the problems of unsatisfactory transfection efficiency and reduced cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
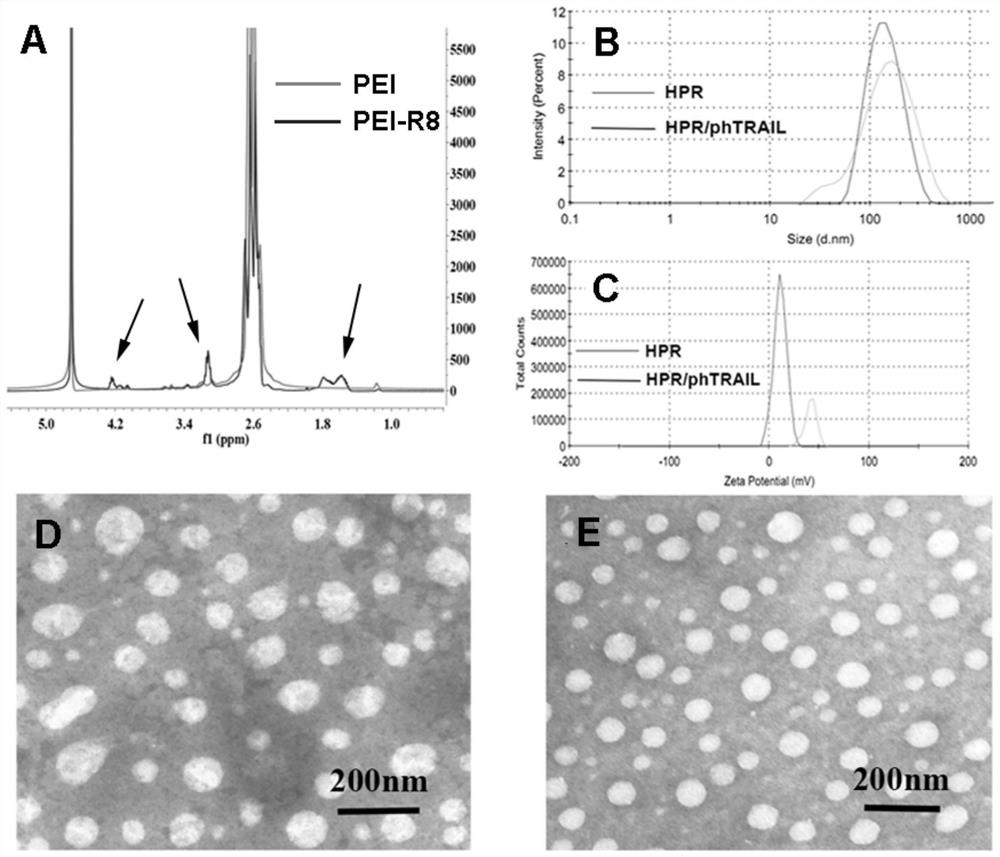
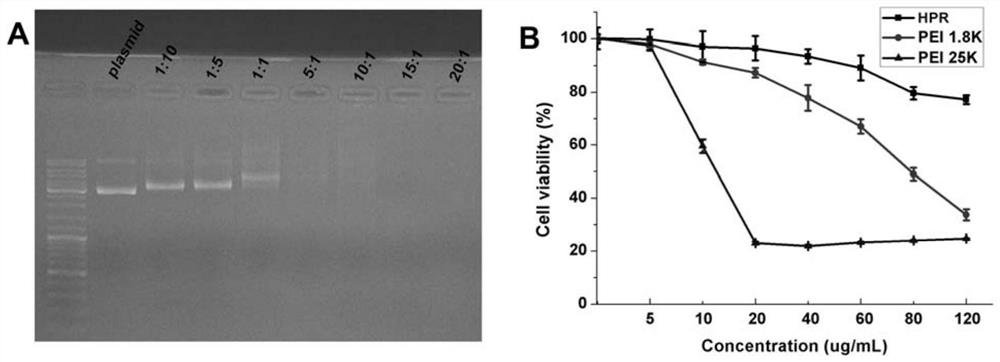
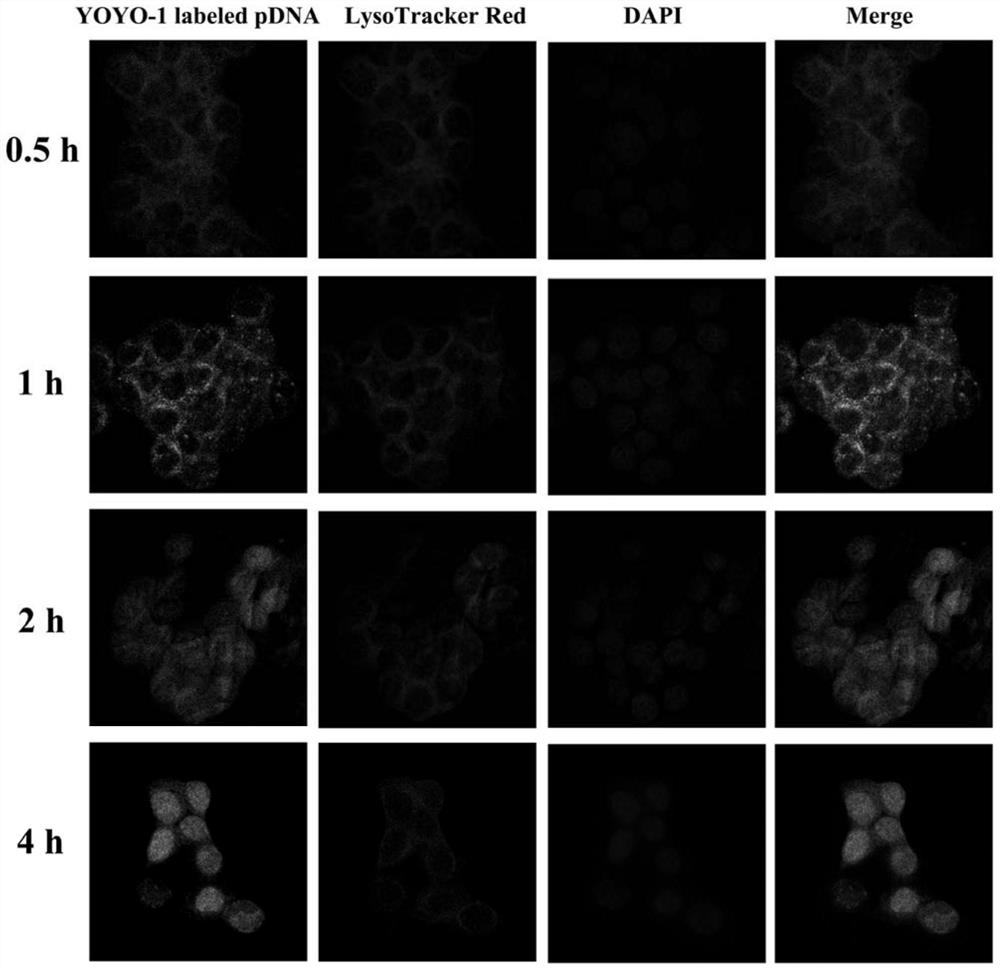
[0028] Embodiment 1 The present invention is used for the preparation of the non-viral gene carrier (HPR) of gene delivery
[0029] 1. Preparation method
[0030] (1) Preparation of PEI-R8
[0031] Dissolve PEI 1.8K (1 g, 0.55 mmol, 1.0 eq) in 10 mL of chloroform in a 25 mL round bottom flask, then add 3-maleimido propionate hydroxysuccinimide ester (177 mg, 0.666 mmol, 1.2 eq). The solvent was removed after the reaction was stirred at room temperature for 3 hours. Then with 10mL THF / H 2 O (1:1, v / v) dissolved the above residue, then added cys-R8 (760mg, 0.55mmol, 1.0eq) and stirred at room temperature for 6 hours. After the reaction was complete, the above reaction solution was dialyzed in water for three days with a dialysis bag with a molecular weight cut off of 1000. Finally, the aqueous solution of the obtained product was lyophilized and stored at -20°C for future use.
[0032] (2) Preparation of HPR
[0033] Dissolve 50 mg of heparin sodium in 100 mL of MES buffe...
Embodiment 2
[0040] Embodiment 2 The present invention is used for the preparation of the non-viral gene carrier (HPR) of gene delivery
[0041] 1. Preparation method
[0042] (1) Preparation of PEI-R8
[0043] Dissolve PEI 1.8K (1 g, 0.55 mmol, 1.0 eq) in 10 mL of chloroform in a 25 mL round bottom flask, then add 3-maleimido propionate hydroxysuccinimide ester (177 mg, 0.666 mmol, 1.2 eq). The solvent was removed after the reaction was stirred at room temperature for 3 hours. Then with 10mL THF / H 2 O (1:1, v / v) dissolved the above residue, then added cys-R8 (760mg, 0.55mmol, 1.0eq) and stirred at room temperature for 6 hours. After the reaction was complete, the above reaction solution was dialyzed in water for three days with a dialysis bag with a molecular weight cut off of 1000. Finally, the aqueous solution of the obtained product was lyophilized and stored at minus 20 degrees for future use.
[0044] (2) Preparation of HPR
[0045] Dissolve 50 mg of heparin sodium in 100 mL o...
Embodiment 3
[0046] Embodiment 3 The present invention is used for the preparation of the non-viral gene carrier (HPR) of gene delivery
[0047] 1. Preparation method
[0048] (1) Preparation of PEI-R8
[0049] Dissolve PEI 1.8K (1 g, 0.55 mmol, 1.0 eq) in 10 mL of chloroform in a 25 mL round bottom flask, then add 3-maleimido propionate hydroxysuccinimide ester (177 mg, 0.666 mmol, 1.2 eq). The solvent was removed after the reaction was stirred at room temperature for 3 hours. Then with 10mL THF / H 2 O (1:1, v / v) dissolved the above residue, then added cys-R8 (760mg, 0.55mmol, 1.0eq) and stirred at room temperature for 6 hours. After the reaction was complete, the above reaction solution was dialyzed in water for three days with a dialysis bag with a molecular weight cut off of 1000. Finally, the aqueous solution of the obtained product was lyophilized and stored at minus 20 degrees for future use.
[0050] (2) Preparation of HPR
[0051] Dissolve 50 mg of heparin sodium in 100 mL o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com