Application of isopentenyl dihydroflavonoids in the field of prevention and treatment of bone diseases
A technology of prenyl dihydroflavonoids and compounds, applied in the preparation of bone disease prevention and treatment drugs and health care products, in the field of prenyl dihydroflavonoids, can solve the problem of curative effect, unclear side effects, and difficult quality control , the mechanism of action is not clear, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The preparation method of compound 1 and compound 2 comprises the following steps:
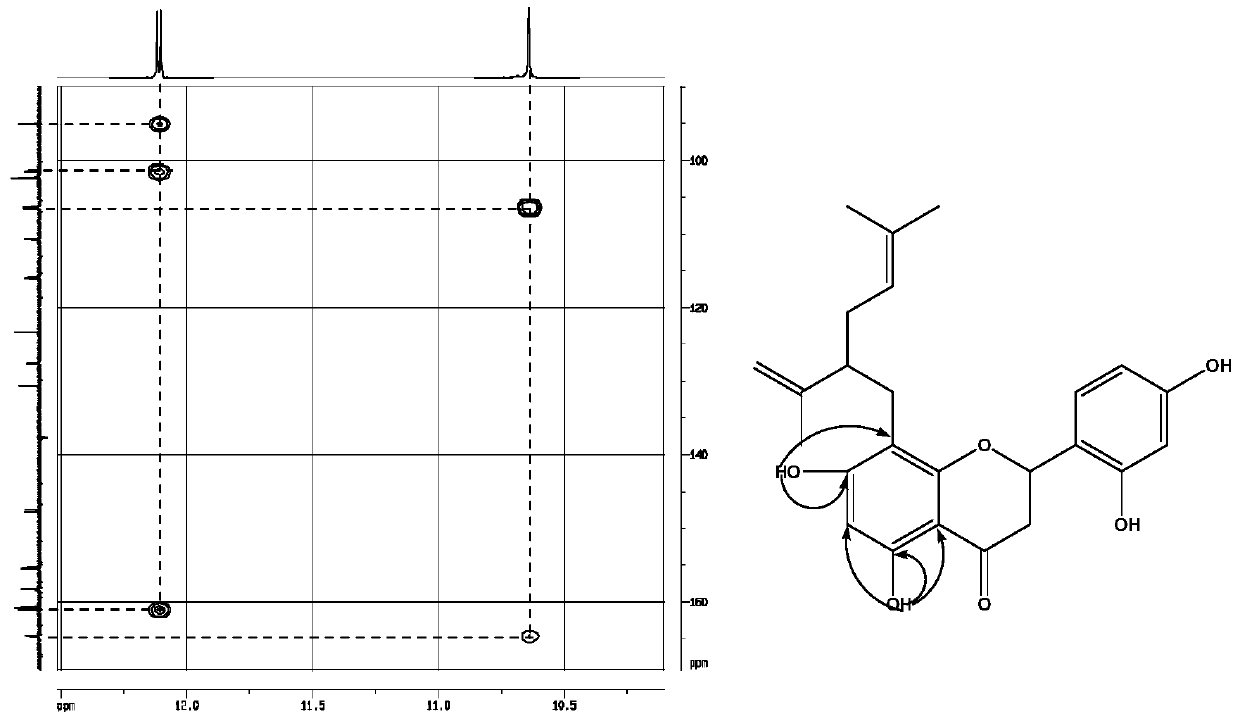
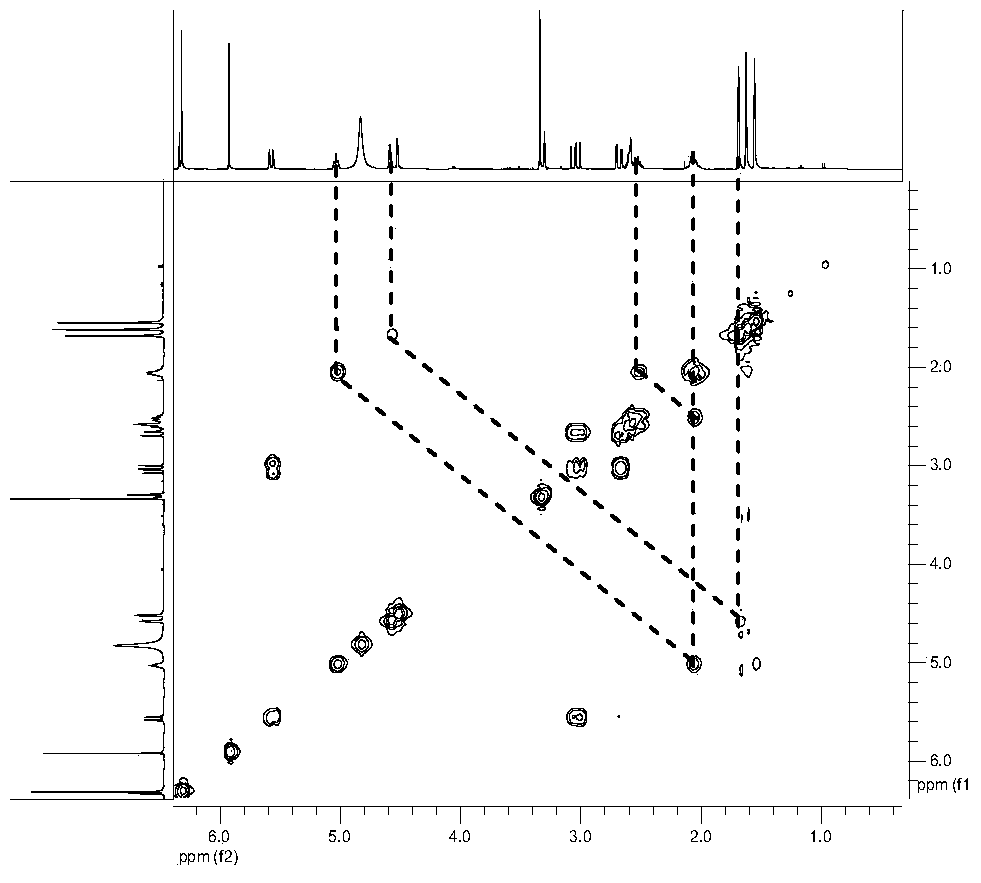
[0054] S11. Take 50 kg of rhizome of Quercus fern (DF), heat and reflux extraction with 8 times the amount of 60% ethanol for 2 hours, extract twice in total, evaporate the solvent to 37L under reduced pressure (take 100mL, evaporate to dryness under reduced pressure, weigh about 10.3 g, equivalent to the total extract (DF) 3.8kg, take out 2000mL, evaporate to dryness, freeze-dry, and keep it for animal experiments). Steam until the macroporous resin on the alcohol-free liquid extract (¢25×150cm);
[0055] S12. Use 0, 30% (v / v), 50% (v / v), 70% (v / v), 95% (v / v) ethanol water to elute sequentially (about 200L / gradient), The 95% ethanol elution fraction of the solvent was recovered under reduced pressure, and a total of 72 g was obtained (shown by activity screening that this fraction has anti-osteoporosis activity);
[0056] S13. Take 15 g of 95% ethanol eluting part and separate it wit...
Embodiment 2
[0075] Effects of compound 1 and compound 2 obtained in Example 1 on the catalytic activity of cathepsin K.
[0076] The Cathepsin K inhibitor screening kit was used for verification. Since Cathespin K can selectively cut off the linkage between its polypeptide substrate and AFC, a fluorescent AFC fragment product can be generated. By detecting the fluorescence intensity of its degradation product AFC, the test compound Inhibition of Cathepsin K protein activity for quantitative detection. Dissolve Cathepsin K (Human Recombinant) protein with reaction buffer, prepare Cathepsin K protein solution, mix and evenly add to black 96 microwell plate at the ratio of 1 μl Ctsk protein solution, 1 μl Ctsk reaction solution, and 48 μl reaction buffer per well . Then 10 μl of the positive drug compound FF-FMK at the concentration to be tested and different concentrations of compound 1 (expressed as SG) and compound 2 (expressed as KF) were added, mixed evenly with the protein mixture, an...
Embodiment 3
[0082] Effects of Compound 1 and Compound 2 on the Survival of Osteoclast Precursor Cells, Macrophage Cell Line RAW264.7
[0083] The effect of compound 1 and compound 2 isolated in Example 1 on the survival of osteoclast precursor cells was evaluated by the cell proliferation activity of osteoclast precursor cell macrophage cell line RAW264.7.
[0084] RAW264.7 cells were inoculated in 96-well plates (1×104 cells / well), cultured in DMEM medium containing 10% FBS (fetal bovine serum) and 1% antibiotics, and after 24 hours (37°C, humidity 95 %, 5% CO2).
[0085] One day later, different concentrations of compound 2 (5-50 μM), compound 1 (5-50 μM), negative control group (1% ethanol) were added and replaced with new culture medium, co-cultivated for four days, and replaced with a new medium every two days. culture medium and drugs.
[0086] After the fourth day, discard the original nutrient solution, and prepare 0.2mg / ml 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxybenzene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com