A kind of cyano complex compound and its preparation method and application
A technology of complex compound and cyano group, applied in the field of cyano complex compound and its preparation, can solve the problems of unsatisfactory cycle stability, poor crystallinity of ferrocyanide, low capacity of sodium ion battery, etc., and achieve good crystallinity , the effect of short cycle and high capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
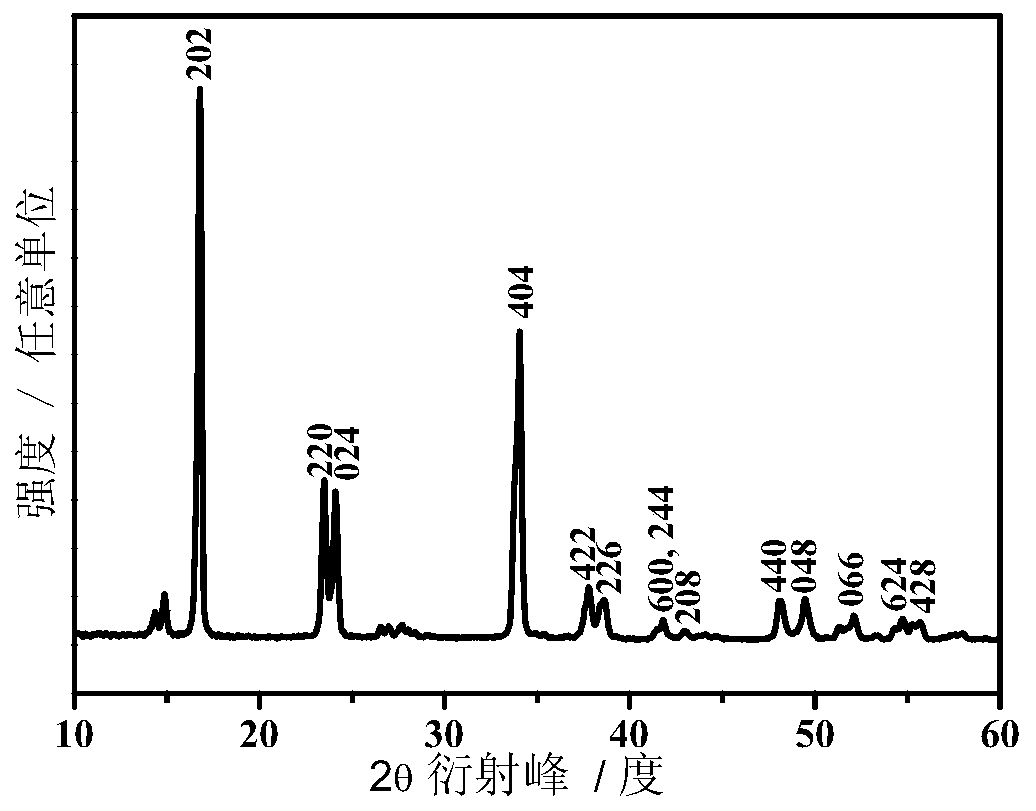
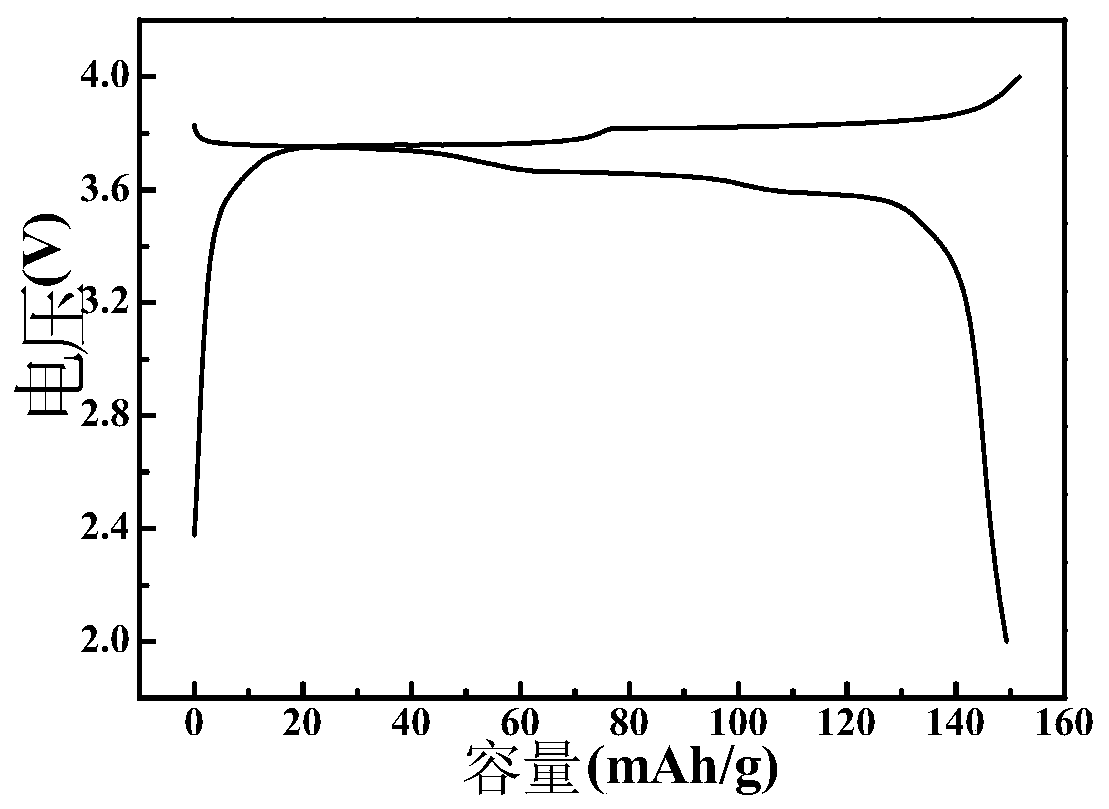
[0035] Dissolve ammonium ferrocyanide in deionized water, stir evenly to obtain ferrocyanide ion (Fe(CN) 6 4- ) meter concentration is the solution A of 0.1mol / L; MnCl 2 Dissolved in deionized water to obtain Mn 2+ Solution B (MnCl 2 The molar ratio of ammonium ferrocyanide to ammonium ferrocyanide is 2.5); solution A and solution B were mixed, subjected to hydrothermal reaction at 60°C for 2 hours, filtered, washed and dried to obtain (NH 4 ) x Mn[Fe(CN)] y Precipitation; NaCl is dissolved in the mixed solvent of ethylene glycol and deionized water (the volume ratio of the two is 90:10) to obtain a concentration of 0.2mol / L solution C (the mol ratio of NaCl and ammonium ferrocyanide is 2.5 ), will (NH 4 ) x Mn[Fe(CN)] y Add it into solution C, after ultrasonic dispersion, under nitrogen atmosphere, react at 150°C for 1.5h, then cool, filter, wash and dry to obtain the cyano complex compound Na x Mn[Fe(CN)] y . Through ICP analysis, the x value of the product is 1.7...
Embodiment 2
[0045] Dissolve ammonium ferrocyanide in deionized water, stir evenly to obtain ferrocyanide ion (Fe(CN) 6 4- ) solution A with a concentration of 0.2mol / L; MnCl with a molar ratio of 1:1 2 and FeSO 4 Dissolved in deionized water to obtain Mn 2+ and Fe 2+ Solution B (MnCl 2 with FeSO 4 The ratio of the total moles of ammonium ferrocyanide to the moles of ammonium ferrocyanide is 3); solution A and solution B are mixed, subjected to hydrothermal reaction at 60°C for 2 hours, filtered, washed and dried to obtain (NH 4 ) x mn 0.5 Fe 0.5 [Fe(CN)] y Precipitation; NaCl is dissolved in the mixed solvent of ethylene glycol and deionized water (the volume ratio of the two is 90:10) to obtain a concentration of 0.4mol / L solution C (the mol ratio of NaCl and ammonium ferrocyanide is 3 ), will (NH 4 ) x mn 0.5 Fe 0.5 [Fe(CN)] y Add it into solution C, after ultrasonic dispersion, under nitrogen atmosphere, react at 140°C for 2h, then cool, filter, wash and dry to obtain th...
Embodiment 3
[0048] Dissolve ammonium ferrocyanide in deionized water, stir evenly to obtain ferrocyanide ion (Fe(CN) 6 4- ) meter concentration is solution A of 0.1mol / L; FeCl 2 dissolved in deionized water to obtain Fe 2+ Solution B (FeCl 2 The molar ratio of ammonium ferrocyanide to ammonium ferrocyanide is 3); solution A and solution B are mixed, subjected to hydrothermal reaction at 60°C for 2 hours, filtered, washed and dried to obtain (NH 4 ) x Fe[Fe(CN)] y Precipitation; NaCl is dissolved in a mixed solvent of ethylene glycol and deionized water (the volume ratio of the two is 90:10) to obtain a concentration of 0.2mol / L solution C (N a The mol ratio of Cl and ammonium ferrocyanide is 2.5), with (NH 4 ) x Fe[Fe(CN)] y Add it into solution C, after ultrasonic dispersion, under nitrogen atmosphere, react at 160°C for 1 hour, then cool, filter, wash and dry to obtain the cyano complex compound Na x Fe[Fe(CN)] y , where, x=1.72, y=0.83; the lattice structure is rhombohedral p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com