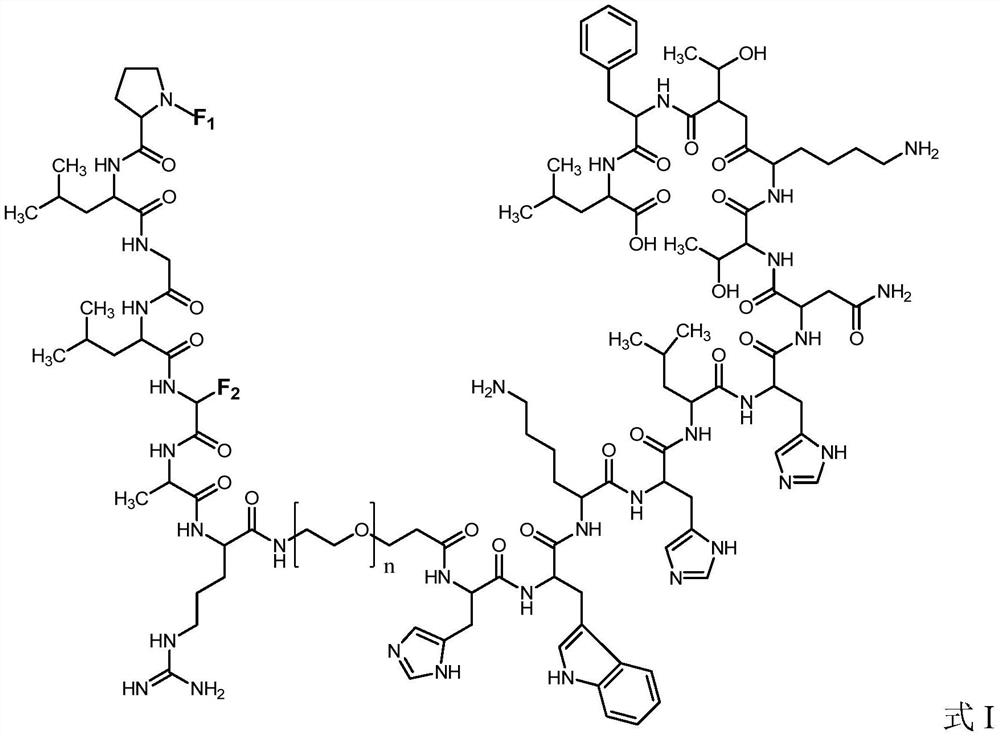
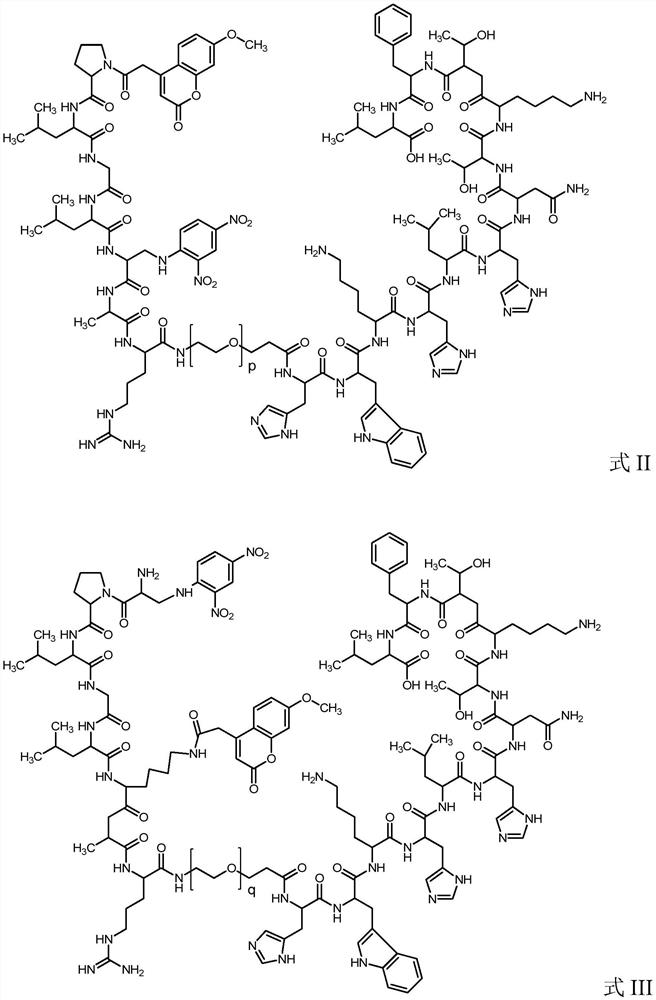
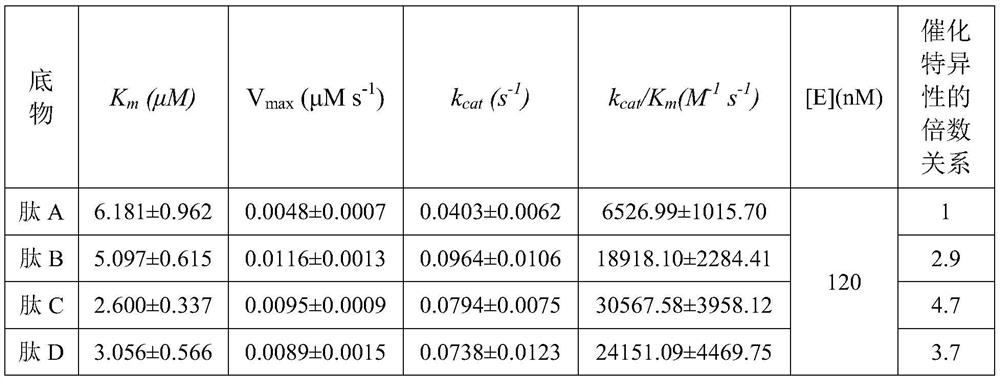
High specificity mmp-14 substrate peptide and its preparation method and application
A MMP-14, high-specificity technology, applied in the field of biological enzyme detection, achieves the effect of simple structure and high safety in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0053] Synthesis sequence: from C-terminal to N-terminal. The synthesis steps are as follows:
[0054] (1) Resin swelling
[0055] Put 2-Chlorotrityl Chloride Resin into the reaction tube, add DCM (15mL / g), and shake for 30min.
[0056] (2) Take the first amino acid
[0057] Filter out the solvent through a sand core, add a 3-fold molar excess of Fmoc-Leu-OH amino acid, add DMF to dissolve, then add a 10-fold molar excess of DIEA, and shake for 60 minutes. Block with methanol.
[0058] (3) Deprotection
[0059] Remove DMF, add 20% piperidine DMF solution (15mL / g) for 5min, remove and add 20% piperidine DMF solution (15mL / g) for 15min.
[0060] (4) Detection
[0061] Take out the piperidine solution, take more than a dozen resins, wash them three times with ethanol, add detection reagents for detection, heat at 105°C-110°C for 5min, and turn dark blue as a positive reaction.
[0062] (5) Washing: DMF (10 mL / g) twice, DCM (10 mL / g) twice, DMF (10 mL / g) twice.
[0063] (6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com