Peptides for use in treatment and diagnosis of type 1 diabetes
一种组合物、氨基酸的技术,应用在疾病诊断、肽、肽源等方向,能够解决T1D无法治愈等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0185] Example 1. Islet autoimmunity in T1D
[0186] The aim of the study was to quantify and compare T cell responses to islet autoantigens, immunodominant gluten peptides, and pathogen-derived recall antigens before and after oral gluten challenge in patients with both type 1 diabetes (T1D) and celiac disease answer.
[0187] background
[0188] Dietary gluten may play a role in inducing or enhancing islet autoimmunity, but the mechanism remains unclear. In patient-based studies over the past 14 years, short-term "gluten challenges" have provided detailed insight into the immune response in celiac disease. In this study, gluten challenge was first used to study patients affected by celiac disease as well as T1D to detect CD4+ T cells specific for self-antigens implicated in T1D and to test whether islet autoimmunity is affected by gluten immunity effect on reactivation.
[0189] In celiac disease, the most prevalent genetic association is with the MHC class II allele e...
example 21
[0279] Example 2. Gluten immunity and islet autoimmunity in type 1 diabetes
[0280] The aim of this study was to quantify and compare T cell responses to islet autoantigens, immunodominant gluten peptides, and pathogen-derived recall antigens before and after oral gluten challenge in patients with both type 1 diabetes (T1D) and celiac disease answer. CD4+ and CD8+ T cell depletion of whole blood samples is performed in order to determine the specific cell populations of T cell cytokine responses to the T1D peptide antigen being analyzed.
[0281] Human CD4+ and CD8+ T cells were depleted directly from whole blood according to the manufacturer's protocol using CD4 and CD8 Dynabeads, respectively (Dynabeads Human CD4, Life Technologies, #11145D; Dynabeads Human CD8, Life Technologies, #11145D) . Briefly, Dynabeads were resuspended and the required volume was transferred to a 15Ml tube. The volume of beads added to whole blood is calculated as 2x 10 per M1 of whole blood 6...
example 3
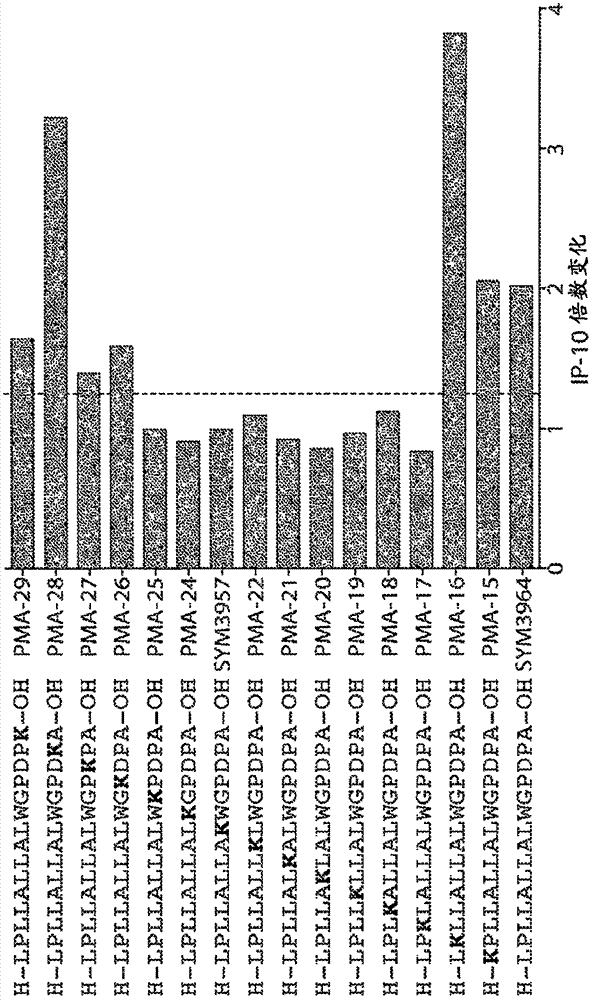
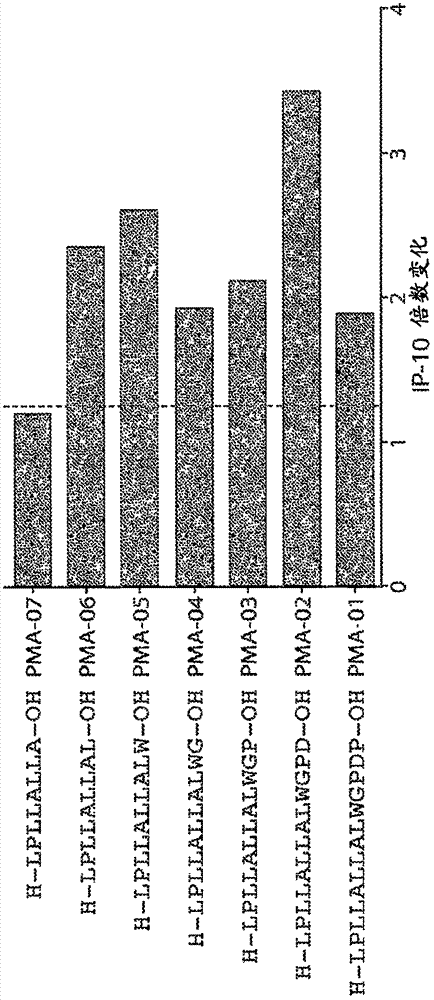
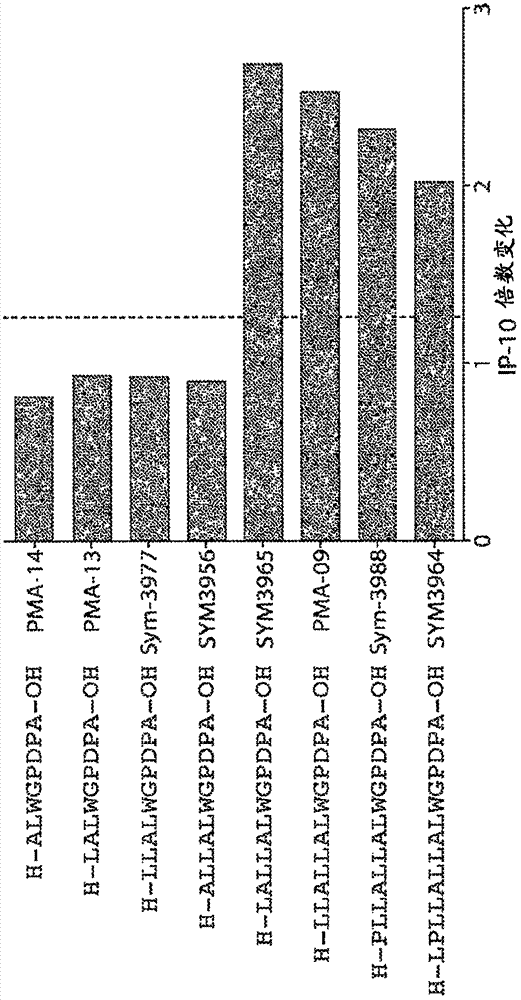
[0310] Example 3. Fine mapping of preproinsulin epitopes
[0311] The leader sequence of the preproinsulin epitope was finely mapped with lysine substituted peptides and C- and N-terminal truncations. Figures 1A-1C Diagram showing the dominant 11-mer epitope in preproinsulin p10-20: LLALLALWGPD (SEQ ID NO: 14). In 4 subjects with T1D, the length and observed biological activity in the whole blood IP-10 release assay was compatible with the epitope of CD4 T cells. However, the activity of the 9-mer p8-16:LPLLALLAL (SEQ ID NO: 76) indicated that this is a CD8 T cell epitope and overlaps with the CD4 epitope. Without wishing to be bound by any theory, therapeutic and / or diagnostic peptides according to the present disclosure may be chosen to avoid CD8 activity, but ensure targeting of CD4 T cells. Data are mean values of whole blood IP-10 release from 4 subjects, measured as fold change relative to medium alone (no peptide).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com