Medicament for inhibiting proliferation of melanoma cells and application thereof
A technique for inhibiting melanin and tumor cells, applied in the fields of biomedicine and melanoma treatment, to achieve the effect of not reducing immunity, increasing apoptosis peak, and obvious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
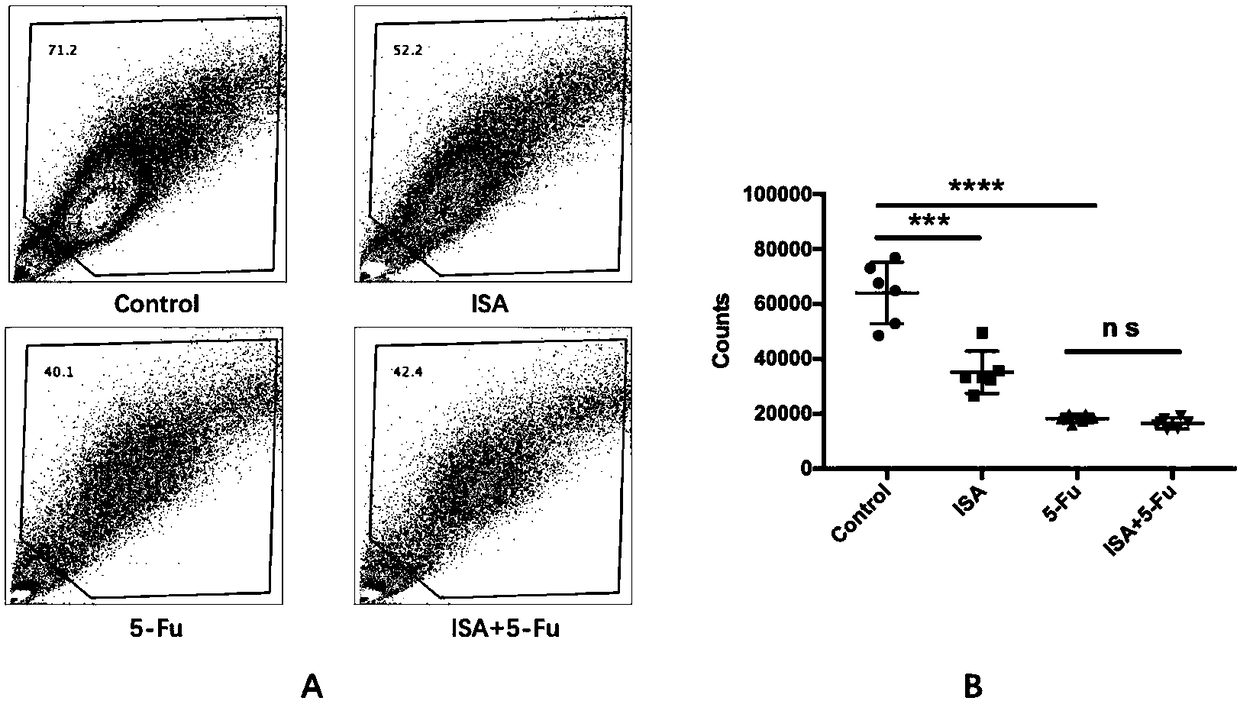
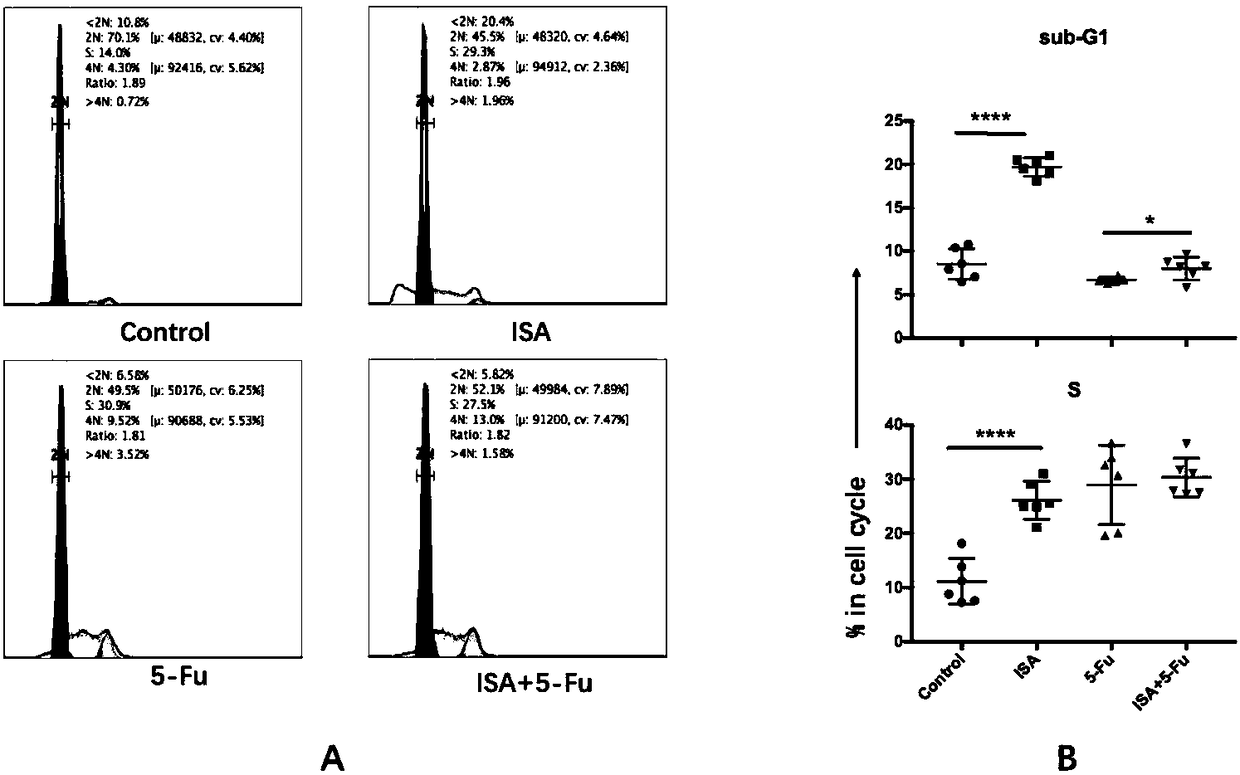
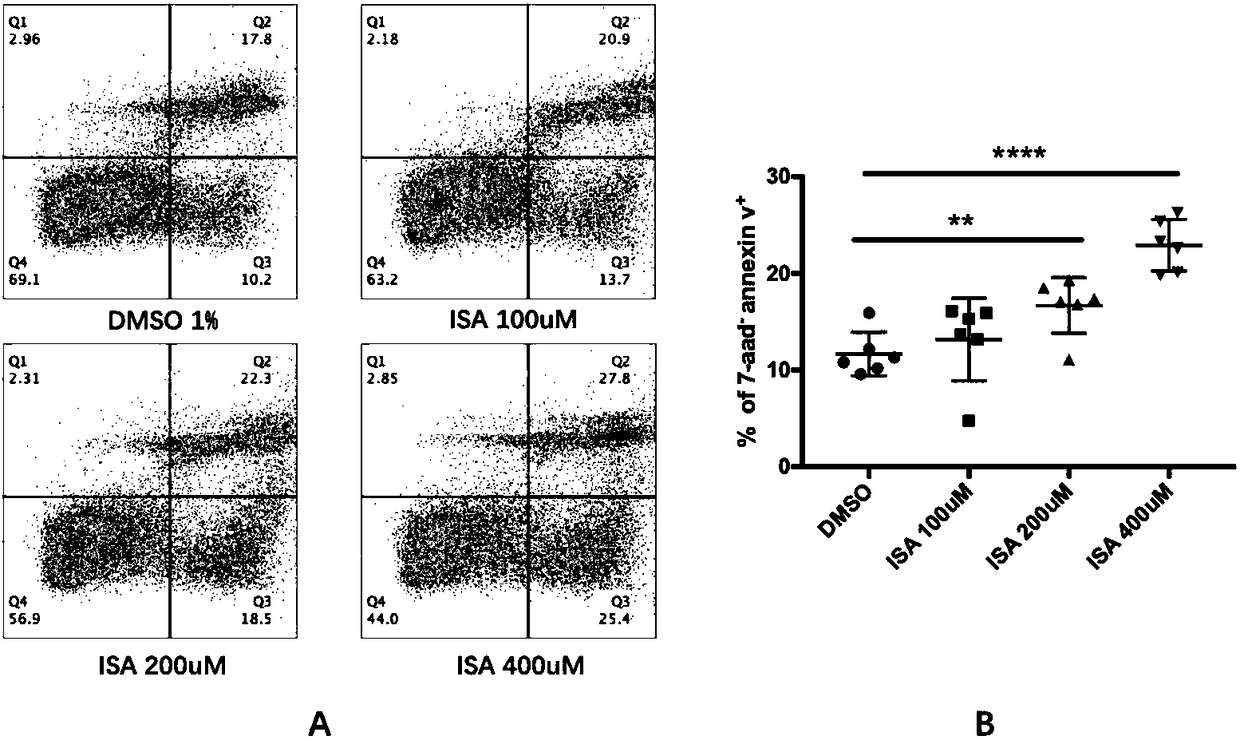
[0023] Embodiment 1-4 is in order to illustrate in vitro cell line experimental data:
[0024] The B16 cell lines were divided into four groups: control group, 2,3-indole quinone group, 5-fluorouracil group and combined drug group. Six wells in each group were treated for 48 hours. Use a flow cytometer with a fixed volume of 300ul / well, the same speed, and a fixed sampling time of 120s / well to detect cell number, cell cycle, apoptosis, and Bcl-2 expression.
[0025] Example 1 is the control group, with 1% DMSO added.
Embodiment 2
[0026] Example 2 is 2,3-indolequinone group, 2,3-indolequinone is prepared into 100mM mother solution with dimethyl sulfoxide (DMSO): the relative molecular mass of 2,3-indolequinone is 147, Therefore, weigh 14.7mg of 2,3-indolequinone and dissolve it in 1ml of DMSO, and the resulting solution is 100mM 2,3-indolequinone mother solution. Diluted to 200uM, 400uM, 800uM respectively with 1640 medium containing 10% fetal bovine serum.
Embodiment 3
[0027] Example 3 is the 5-fluorouracil group. Dissolve 5-fluorouracil with DMSO into a 100mM mother solution: weigh 13mg of 5-fluorouracil powder and dissolve it in 1ml DMSO, and the resulting solution is 100mM 5-fluorouracil mother solution. Dilute to 200uM with 1640 medium containing 10% fetal bovine serum for use.
[0028] Example 4 is a combination drug group, adding 400uM 2,3-indoloquinone and 200uM 5-fluorouracil (containing 1% DMSO).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap