Automatic issuing system of clopidogrel medication related gene test reports
A clopidogrel and genetic testing technology, applied in the field of medical information management, can solve problems such as error-prone, weakened ability, time-consuming and labor-intensive, etc., and achieve the effect of reducing the incidence of errors, saving time and cost, and avoiding manpower
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
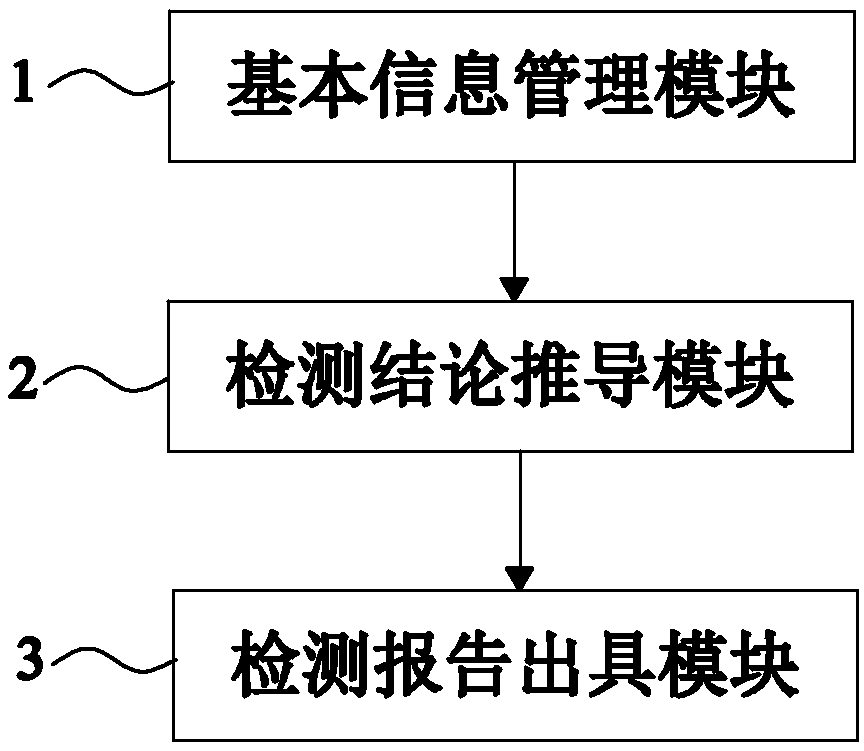
[0098] Such as figure 1 As shown, Embodiment 1 of the present invention provides a system for automatically issuing a gene detection report related to clopidogrel medication, including the following parts:
[0099] The basic information management module 1 is used to sort out and store the basic clinical data of patients and the detection results of genes related to clopidogrel medication. The genes related to clopidogrel medication include CYP2C19 gene, PON1 gene and ABCB1 gene. The CYP2C19 Genes include CYP2C19*2 locus, CYP2C19*3 locus and CYP2C19*17 locus, among which CYP2C19*2 locus, CYP2C19*3 locus and PON1 gene all include GG, GA, AA three genotypes, CYP2C19*17 Both the site and the ABCB1 gene include CC, CT, and TT genotypes;
[0100] The basic clinical information of the patient can be entered manually or exported from the hospital's office system, including medical record number, name, gender, age, ethnicity, height, weight, department, bed number, sending physician,...
Embodiment 2
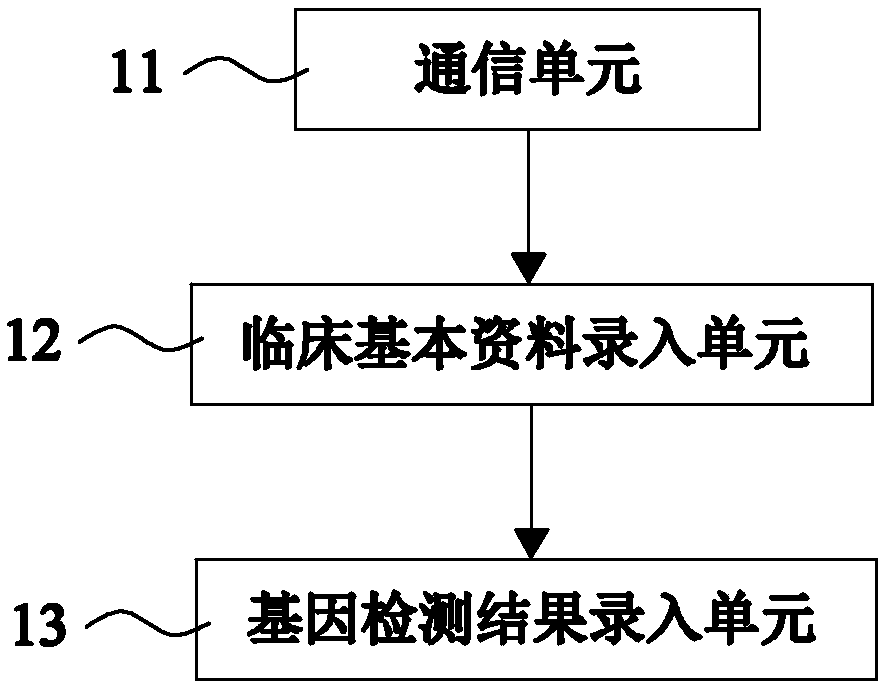
[0107] Such as figure 2 As shown, on the basis of Example 1, this Example 2 provides a system for automatically issuing a clopidogrel drug-related gene detection report. This Example 2 further defines that the basic information management module 1 includes the following parts:
[0108] The communication unit 11 is used to obtain the patient's case information and gene detection information from the hospital information system;
[0109] The related systems of hospital registration information include HIS system (Hospital Information System, hospital information system), LIS system (Laboratory Information System, laboratory information system), etc. The relevant information of patients can be directly retrieved and copied from the above systems, so that There is no need for manual entry when issuing the test report;
[0110] A basic clinical data entry unit 12, configured to extract and store basic clinical information from the case information;
[0111] The gene detection re...
Embodiment 3
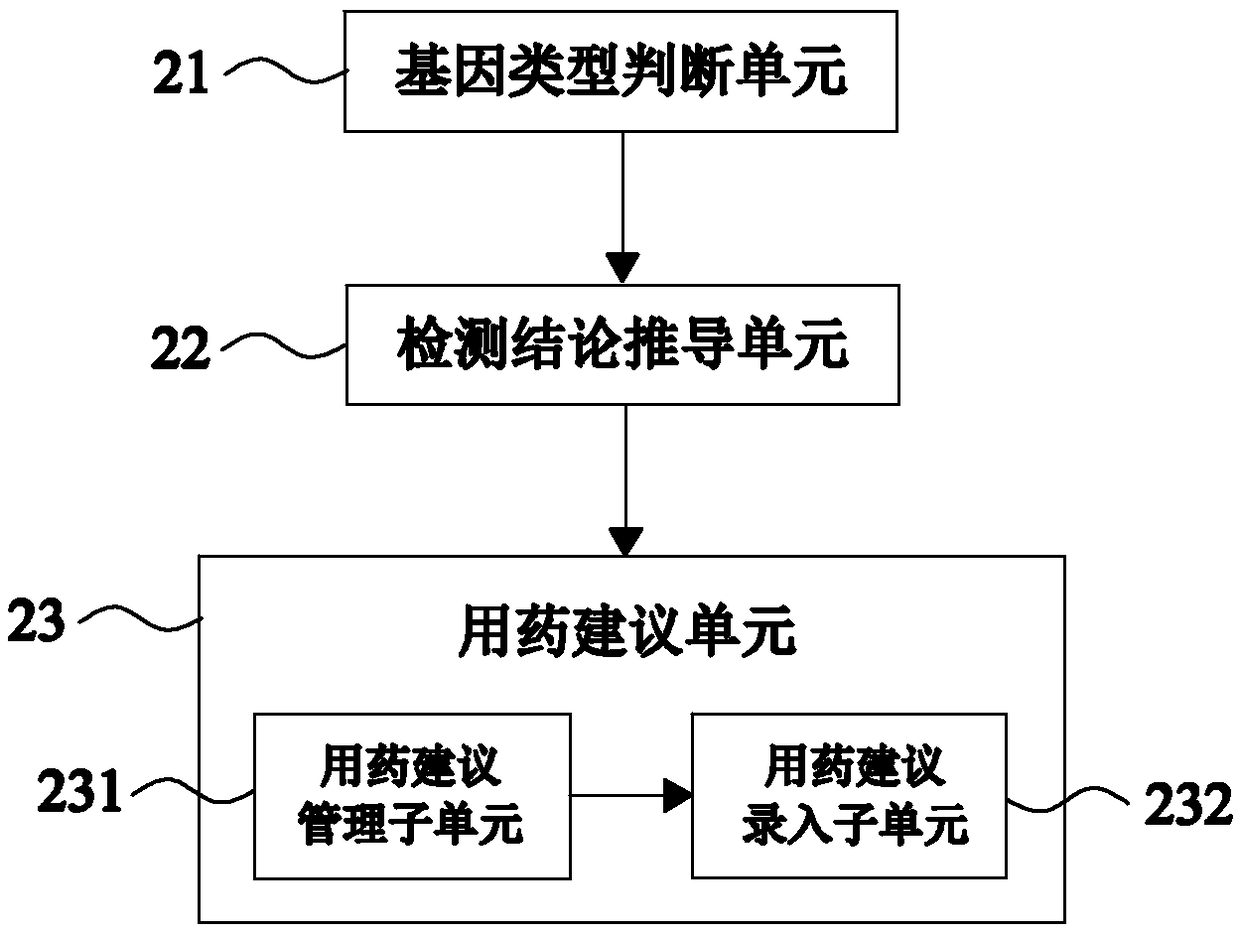
[0115] Such as image 3 As shown, this embodiment 3 provides a system for automatically issuing a clopidogrel drug-related gene detection report on the basis of embodiment 1. This embodiment 3 further defines that the detection conclusion derivation module 2 includes the following parts:
[0116] The gene type judging unit 21 is used to judge the gene types of the patient's CYP2C19 gene, PON1 gene, and ABCB1 gene according to the detection results of clopidogrel-related genes;
[0117] CYP2C19 enzyme is closely related to whether resistance reaction occurs after clopidogrel administration; PON1 enzyme also plays a key role in the process of converting clopidogrel into active product, but its role is not as prominent as CYP2C19 enzyme; Plays a role in intestinal absorption into the blood, but is weaker than the PON1 enzyme. Therefore, it is necessary to judge the genotypes of the above three genes separately, so that the subsequent detection conclusion can be drawn by integrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com