The application of trilensin component b in the preparation of anti-blood stasis syndrome medicine
A technology of blood stasis syndrome and trilens, which is applied in the field of medicine, can solve the problem that the active ingredients of sanlens are not clear enough
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
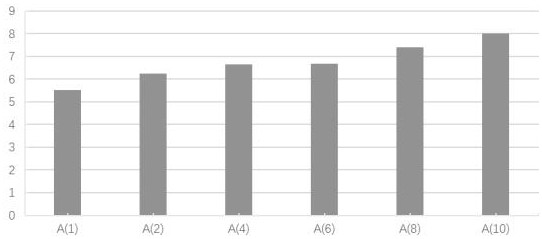
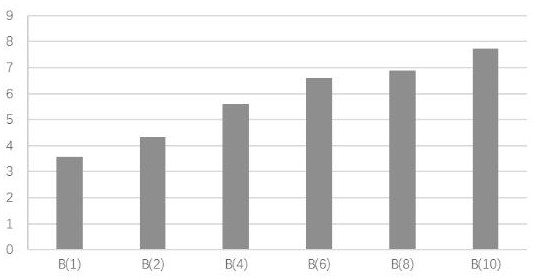
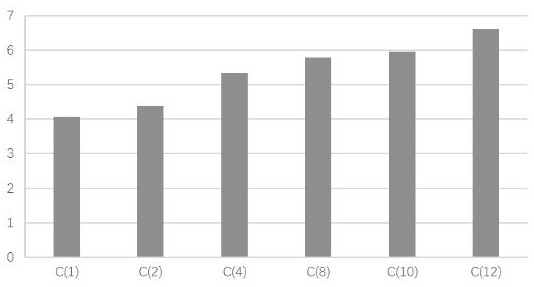
[0025] Example 1 compound blood circulation promoting function experiment
[0026] 1. Experimental materials
[0027] (1) Drugs: Trilens A, Trisensin B and Trisensin C were self-made in the experiment, with a purity of ≥99%.
[0028] (2) Animal: KM mouse, ♀, body weight 25±5g, provided by Guangdong Experimental Animal Center, license number: SCK (Guangdong) 2013-0002.
[0029] (3) Reagents
[0030] Epinephrine Hydrochloride Injection (Grand Medicine (China) Co., Ltd., batch number: 20170214); Bayer Aspirin Enteric-Coated Tablets (Bayer Healthcare Co., Ltd., batch number: BJ32400); Carboxymethylcellulose Sodium (CMC-Na, Sinopharm Chemicals Reagent Co., Ltd., batch number: F20110915, analytically pure); sodium chloride (Tianjin Zhiyuan Chemical Reagent Co., Ltd., batch number: 20160312, analytically pure); mouse thromboxane B2 (TXB2), endothelin 1 (ET-1), Plasminogen activator inhibitor 1 (PAI-1), fibrinogen (FIB) enzyme-linked immunoassay kit (Tianjin Anoricang Biotechnology...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com