Medicine composition containing berberine and sophocarpidine, and purpose of medicine composition for treating or preventing non-alcoholic fatty liver disease
A fatty liver disease, non-alcoholic technology, applied in the field of biopharmaceuticals, can solve the problems of no clear treatment method, no definite treatment effect of liver fibrosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
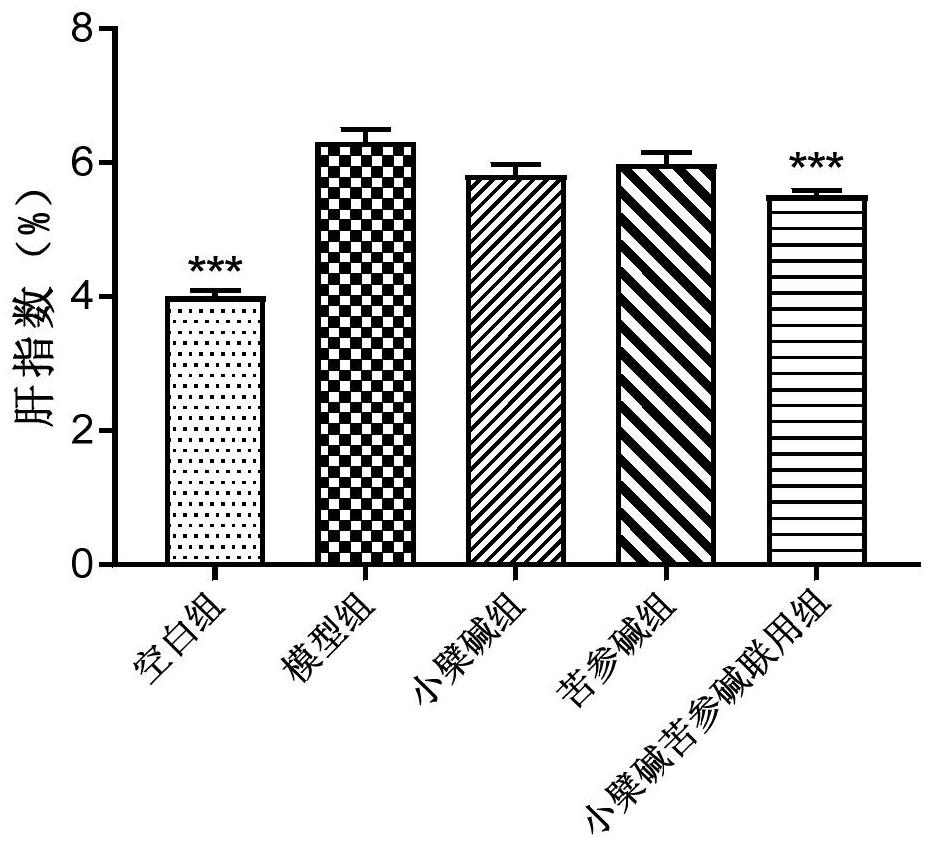
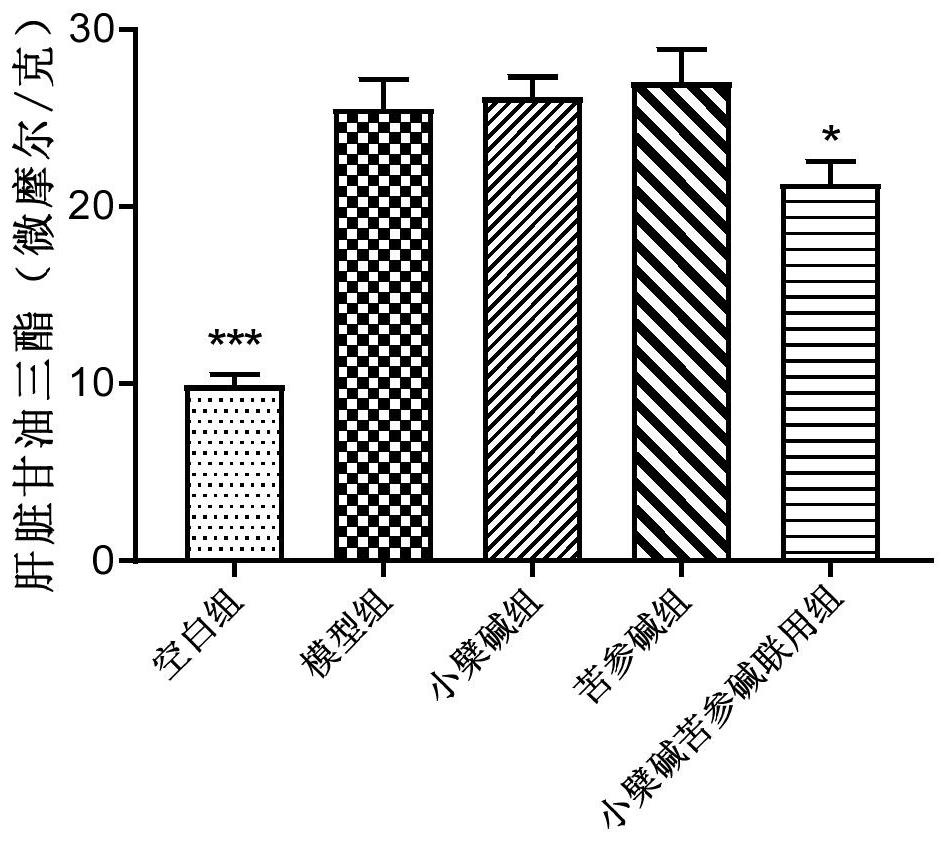
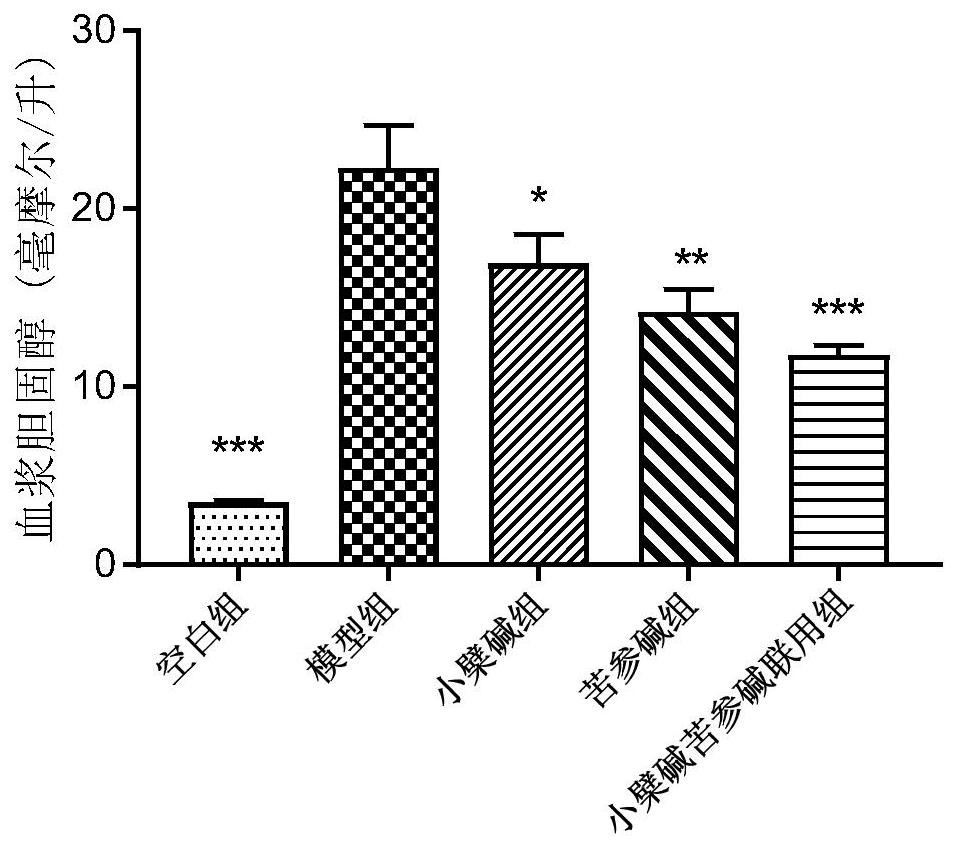
[0090] Experimental method and material
[0091] 1.1 Experimental animals: LVG Syrian golden earth rat, male, 4 weeks old, weight 70-90g, the feeding environment adapted for 2 weeks. Animals were purchased from Beijing Virong Lihua Experimental Animal Technology Co., Ltd.
[0092] 1.2 Experiment Feed: Basic Feed (Normal Chow Diet, White Group); High Fat Diet, Model Group: 1.0% Cholesterol, 0.2% Cholina, 10.0% Laust, 5.0% Egg Yellow Powder and 83.8% Basal Feed ). Feed is purchased from Beijing Keo Cooperation Feed Co., Ltd.
[0093] 1.3 Administration: 1) Preventive Group: Simultaneous administration with high-fat feed, mix the drug in high fat feed, 12 weeks for continuous administration. 2) Treatment group: The high-fat feed was administered after 8 weeks of administration, and it was administered for 10 weeks. Berberine and bitter ginsee were purchased from Nanjing Zelan Biotechnology Co., Ltd.
[0094] 1.4 Experimental grouping and dose of dose:
[0095]
[0096] 1.5 detectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com