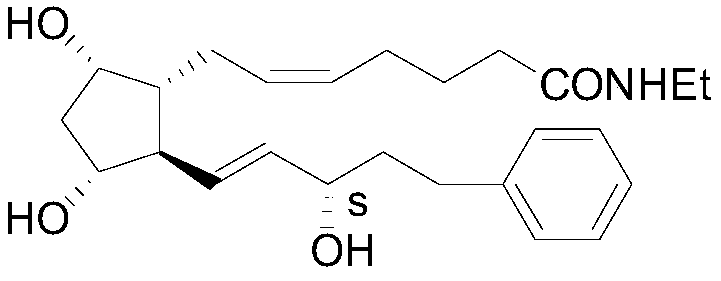
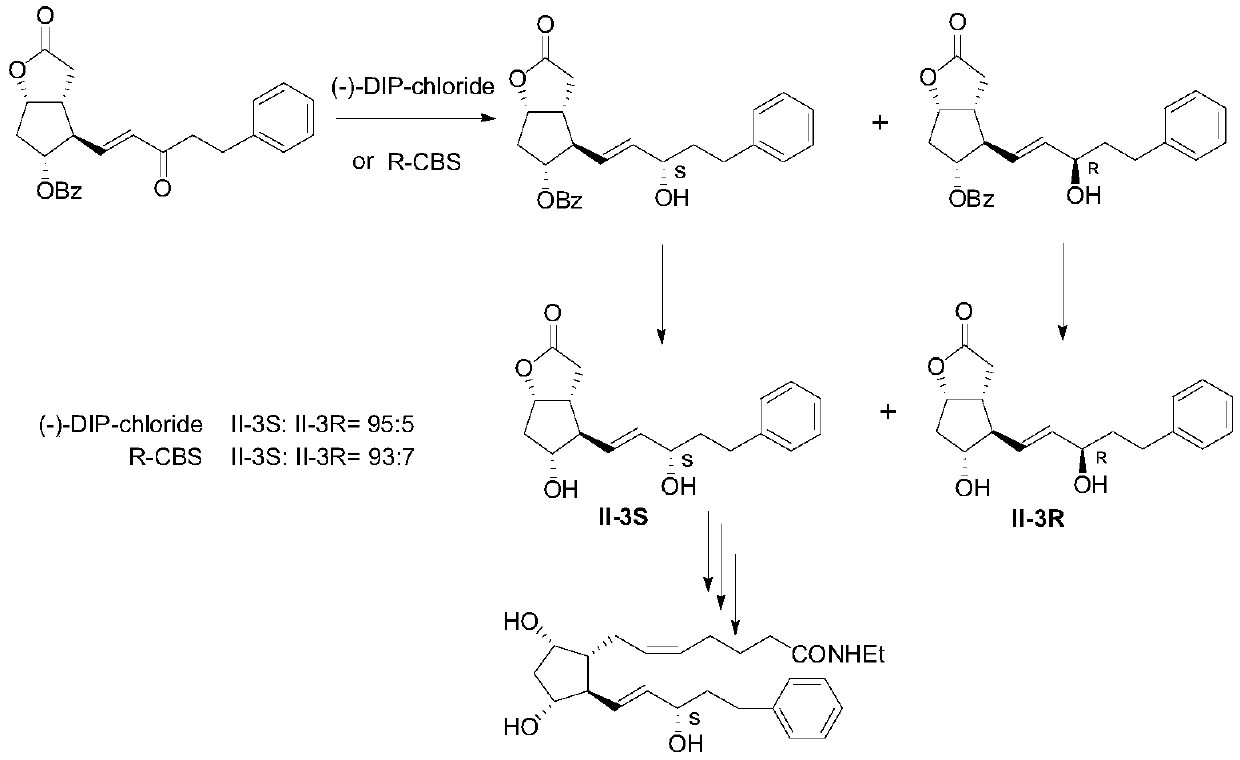
A kind of preparation method of bimatoprost key intermediate
A technology for bimatoprost and intermediates, which is applied in the field of preparation of key intermediates of bimatoprost, and can solve problems such as tediousness, reduced yield, and increased cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
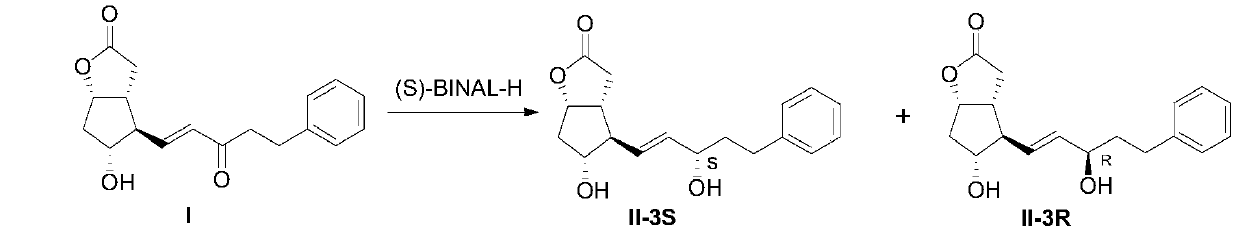
[0053] The preparation method of bimatoprost key intermediate II
[0054] The invention provides a preparation method capable of obtaining bimatoprost key intermediate II (II-3S) with high chiral purity.
[0055] Specifically, the present invention provides a method for preparing bimatoprost key intermediate II, comprising the steps of:
[0056]
[0057] In an inert solvent (such as tetrahydrofuran THF), in the presence of (S)-BINAL-H, the intermediate I is reduced to obtain intermediate II (containing 3S (as shown in formula II-3S) and 3R (as shown in formula II-3S) and 3R (as shown in formula II-3R) two isomers), and S-type isomer II-3S:R-type isomer II-3R>99.5:0.5 (molar ratio).
[0058] Preferably, in the formula, (S)-BINAL-H is a compound shown in formula III
[0059]
[0060] Among them, R is CH 3 or C 2 h 5 .
[0061] Preferably, the preparation method comprises the steps of:
[0062] Step 1: Preparation of (S)-BINAL-H:
[0063] Under the protection of argon...
Embodiment 1
[0074] Step 1: Under the protection of argon, add tetrahydrofuran solution (375.0ml, 0.3mol, concentration 0.8mol / L) of lithium aluminum tetrahydride into the reaction flask, add methanol (9.6g, 0.3mol) in tetrahydrofuran dropwise at 20-30°C (96.0ml) solution, continue to dropwise add S-binaphthol (85.9g, 0.3mol) in tetrahydrofuran (490.0ml) solution, after addition, stir at 20-30°C for 30-60 minutes to obtain (S)-BINAL- H.
[0075] Step 2: Under the protection of argon, cool the (S)-BINAL-H solution in step 1 to -105--95°C, add intermediate I (30.0g, 0.1mol) in tetrahydrofuran (300.0ml) and stir for 30 Minutes later, raise the temperature to -80--70°C and stir until the reduction reaction is complete. Post-treatment, add 50.0ml of methanol, slowly rise to room temperature, filter, add water to the filtrate, stir and separate with ethyl acetate, and wash the water layer with ethyl acetate The ester was back-extracted, the organic layers were combined, washed with saturated br...
Embodiment 2
[0078] Step 1: Under the protection of argon, add tetrahydrofuran solution (375.0ml, 0.3mol, concentration 0.8mol / L) of lithium aluminum tetrahydride into the reaction flask, and add ethanol (13.8g, 0.3mol) in tetrahydrofuran dropwise at 20-30°C (96.0ml) solution, continue to dropwise add S-binaphthol (85.9g, 0.3mol) in tetrahydrofuran (490.0ml) solution, after addition, stir at 20-30°C for 30-60 minutes to obtain (S)-BINAL- H.
[0079] Step 2: Under the protection of argon, cool the (S)-BINAL-H solution in step 1 to -105--95°C, add intermediate I (30.0g, 0.1mol) in tetrahydrofuran (300.0ml) and stir for 30 Minutes later, raise the temperature to -80--70°C and stir until the reduction reaction is complete. Post-treatment, add 50.0ml of methanol, slowly rise to room temperature, filter, add water to the filtrate, stir and separate with ethyl acetate, and wash the water layer with ethyl acetate The ester was back-extracted, the organic layers were combined, washed with saturate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com