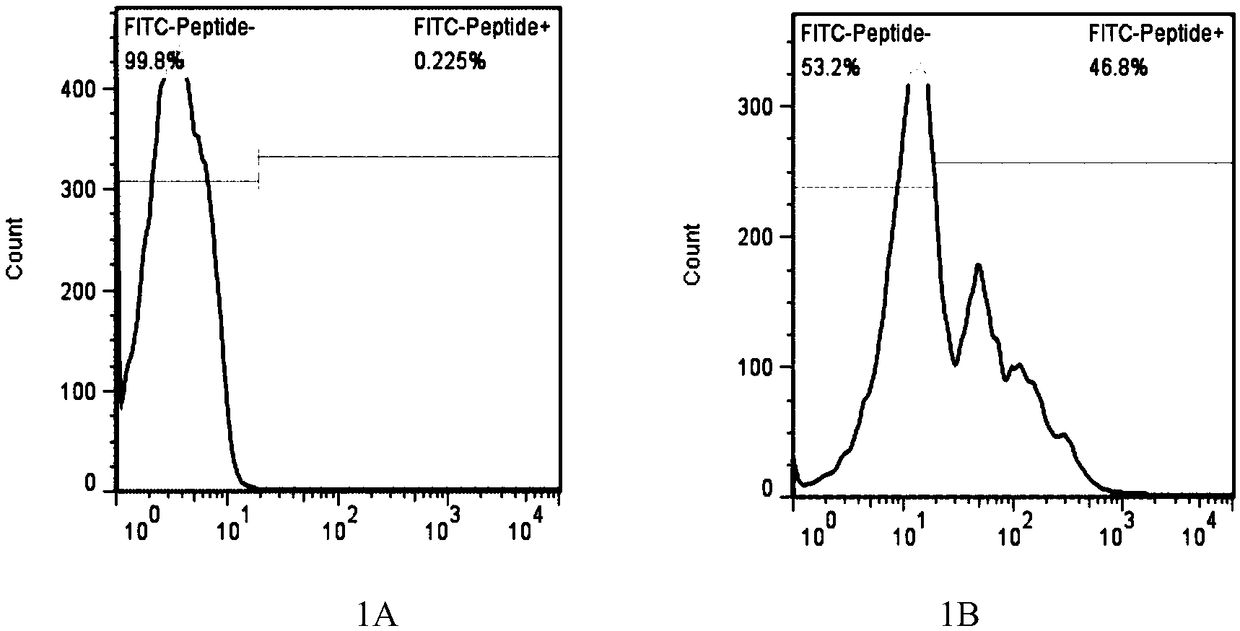
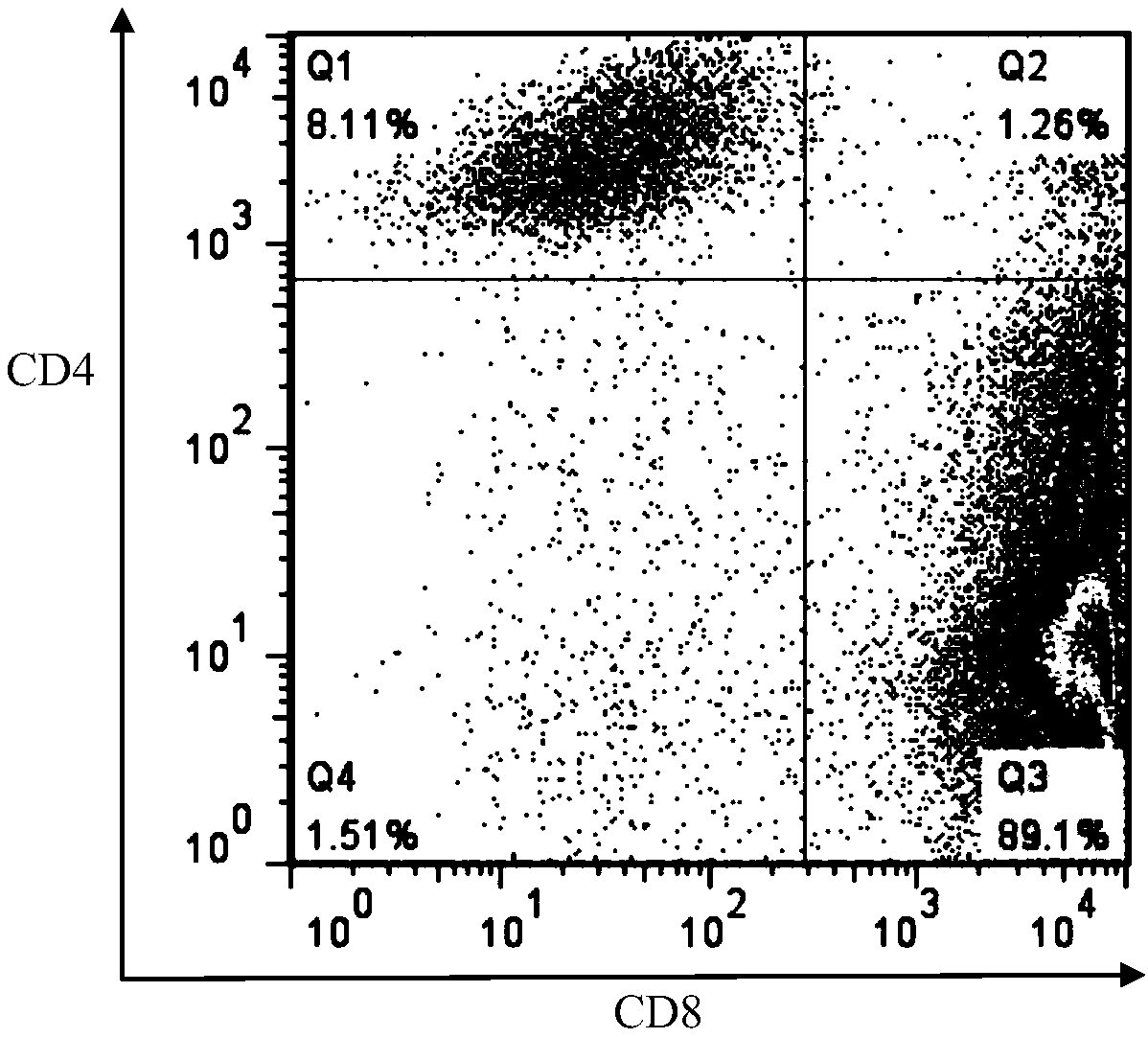
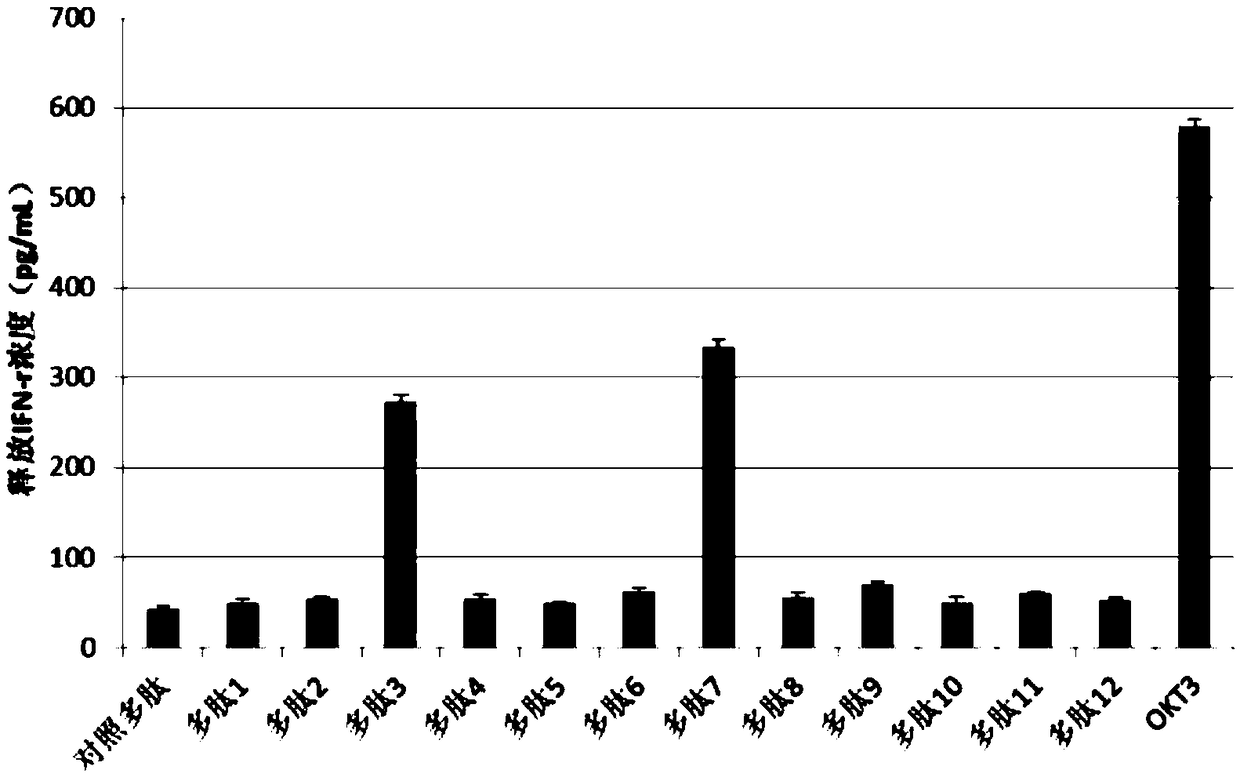
LRFF cells
A cell and antigen technology, applied in the field of LRFF cells and their preparation, can solve the problems of single target, unknown tumor antigen, low efficiency, etc., and achieve the effect of strong target specificity, less off-target effect, and improved lethality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] The present invention is described below through specific embodiments. Unless otherwise specified, the technical means used in the present invention are methods known to those skilled in the art. In addition, the embodiments should be considered as illustrative rather than limiting the scope of the invention, the spirit and scope of which is defined only by the claims. For those skilled in the art, on the premise of not departing from the spirit and scope of the present invention, various changes or modifications to the material components and dosage in these embodiments also belong to the protection scope of the present invention.
[0048] The technical solution is detailed as follows:
[0049] 1. Epitope prediction
[0050] 1) Use the peripheral blood of lung cancer patients for ctDNA sequencing and HLA typing detection;
[0051] 2) Use software to analyze the sequencing information: compare the ctDNA sequencing results with the genome of normal cells, and screen o...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap