A rhodamine-like acylhydrazone derivative, its preparation method and application, and a fluorescent probe
A technology of fluorescent probes and derivatives, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of limited application, high cost, complex synthesis, etc., and achieve the effect of fluorescence differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The present invention provides the preparation method of rhodamine acylhydrazone derivative described in the scheme, comprising the following steps:
[0033] Rhodamine 6G hydrazide, 4-morpholino benzaldehyde and an alcohol solvent are mixed for condensation reaction to obtain rhodamine acylhydrazone derivatives with the structure shown in formula I.
[0034] In the present invention, the molar ratio of the rhodamine 6G hydrazide and 4-morpholino benzaldehyde is preferably 1:1~1.1, more preferably 1:1; the alcohol solvent is preferably ethanol; the rhodamine The volume ratio of the amount of Ming 6G hydrazide to the alcohol solvent is preferably 0.01-0.02 mol: 0.3-0.6 L, more preferably 0.01 mol: 0.3-0.5 L.
[0035] In the present invention, the rhodamine 6G hydrazide is preferably dissolved in an alcohol solvent, and then 4-morpholinobenzaldehyde is added into the solution. In a specific embodiment of the present invention, it is preferable to add 2 to 3 drops of aceti...
Embodiment 1
[0047] Dissolve 428mg of rhodamine 6G hydrazide in 30mL of ethanol, then add 191mg of 4-morpholinobenzaldehyde, add 2 drops of acetic acid under normal pressure to catalyze reflux and stir for 3h, cool to room temperature, precipitate a solid, and filter under reduced pressure. The filter residue was washed with ethanol to obtain a white solid which was the target product, and the yield of the target product was 89%.
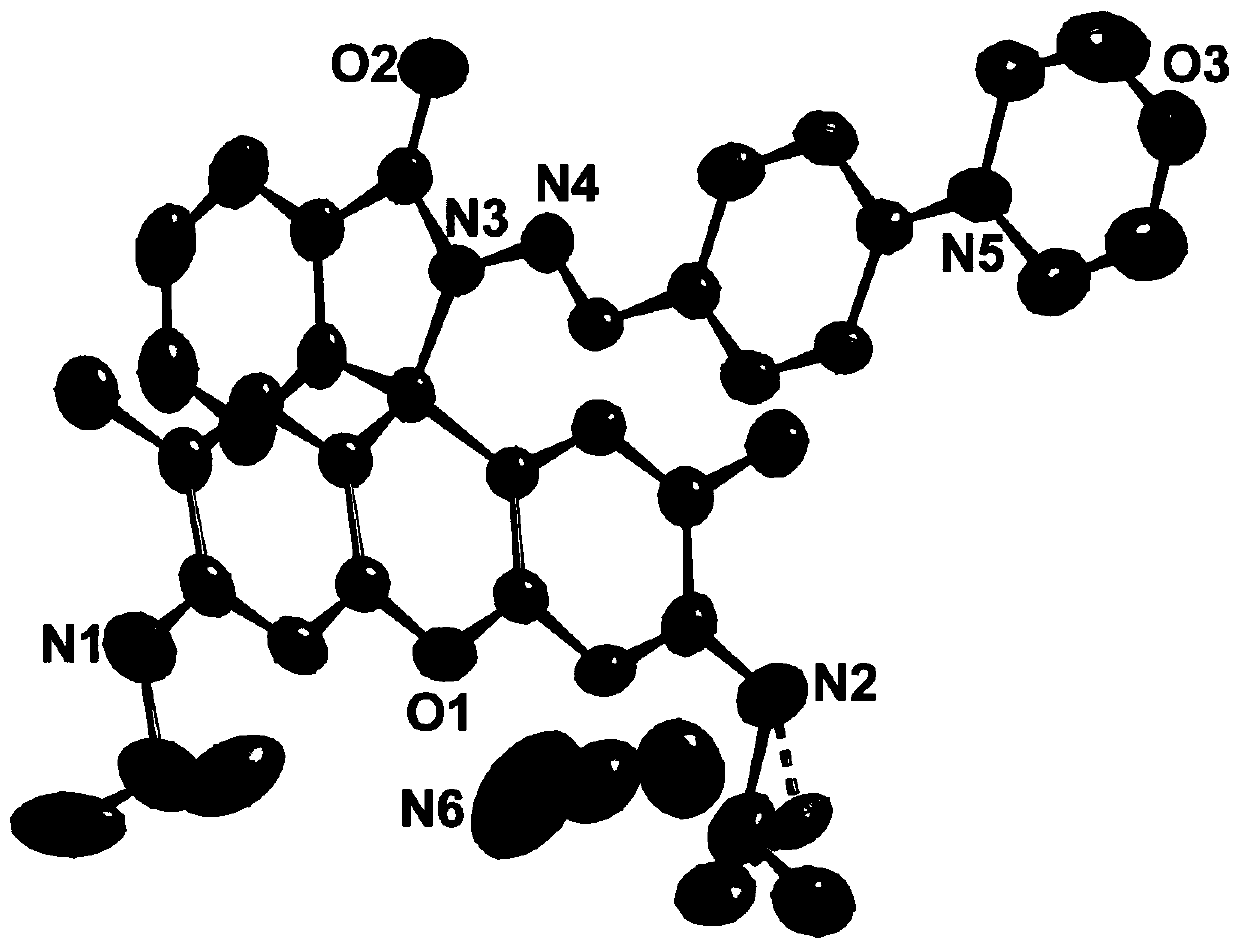
[0048] The resulting product is recrystallized with acetonitrile, and the resulting recrystallized product is subjected to single crystal diffraction analysis to determine the crystal structure of the resulting product. The obtained results are as follows figure 1 Shown; The crystal structure shows that the obtained rhodamine acylhydrazone derivative exists in the form of acetonitrile.
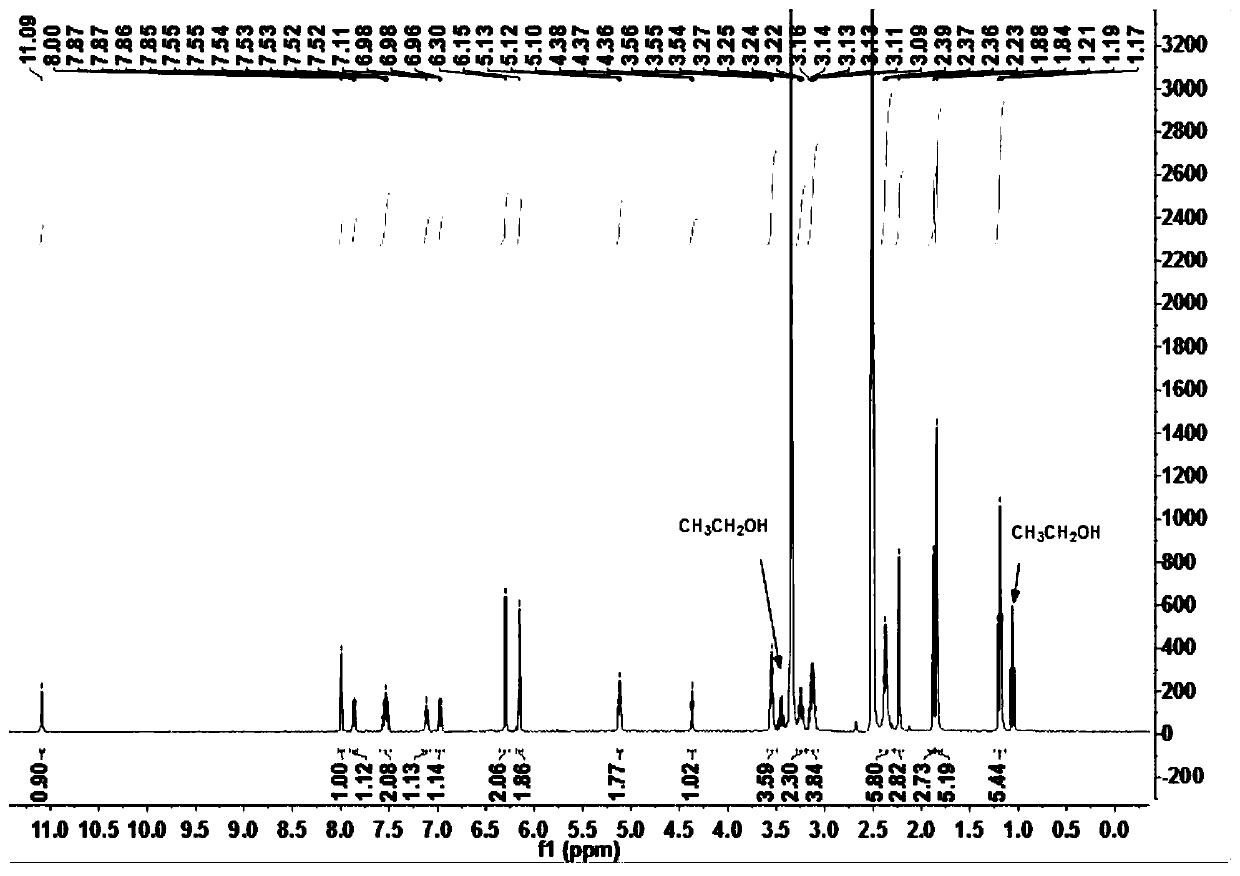
[0049] The obtained rhodamine class acylhydrazone derivatives are carried out nuclear magnetic resonance analysis, and the results are as follows:
[0050] 1 H NMR (400MHz, DM...
Embodiment 2
[0053] The rhodamine-like acylhydrazone derivatives prepared in Example 1 were tested for fluorescence properties in buffer solutions with different pH values, the steps are as follows:
[0054] (1) The rhodamine acylhydrazone derivatives prepared in Example 1 are formulated into molar concentrations in different pH values (1.0~6.0) disodium hydrogen phosphate-citric acid buffer solution (containing 0.5% DMSO by volume ratio) 1×10 -5 mol / L solution, adopt fluorescence spectrometer to carry out fluorescence spectrum analysis respectively (excitation wavelength is 480nm), the fluorescence spectrum figure of gained is shown in Figure 4 . pass Figure 4 It can be seen that the rhodamine-like acylhydrazone derivatives prepared in Example 1 of the present invention have no obvious fluorescence emission as fluorescent probes at 4.0
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com